1 Introduction
It has been demonstrated that ultrastructural studies of the male gamete in Platyhelminthes reveal significant characters for phylogenetic purposes [1–8]. In Pronocephaloidea, data for the ultrastructure of spermatozoa are available only in two of the six families recognized by Barton and Blair [9]. These are Notocotylidae and Pronocephalidae with respective studies in Notocotylus neyrai [10] and in Cricocephalus albus [11].
In the present study, we describe the ultrastructure of the spermatozoon of another Pronocephaloidean, Pleurogonius truncatus, belonging to the same family (Pronocephalidae) that C. albus.
2 Materials and methods
Adult specimens of P. truncatus were collected from the stomach of a specimen of Eretmochelys imbricata recovered dead on a beach of the Atlantic Ocean, near Dakar (Senegal). Live worms were rinsed with a 0.9% NaCl solution and fixed in cold (4 °C) 2.5% glutaraldehyde in a 0.1 M sodium cacodylate buffer at pH 7.2, rinsed in 0.1 M sodium cacodylate buffer at pH 7.2, post-fixed in cold (4 °C) 1% osmium tetroxide in the same buffer for 1 h, rinsed in a 0.1 M sodium cacodylate buffer at pH 7.2, dehydrated in ethanol and propylene oxide, embedded in Spurr's resin and polymerized at 60 °C for 24 h.
Ultrathin sections (60–90 nm thick) were obtained using an Ultramicrotome (Power tome PC, RMC Boeckeler®) with a diamond knife. Sections placed on copper grids were double-stained with uranyl acetate and lead citrate. Sections placed on gold grids were stained with periodic acid, thiocarbohydrazide and silver proteinate [12]. This technique was employed for the location of the glycogen.
The copper grids were examined in a Hitachi H-7650 transmission electron microscope operated at 80 kV, in the “Service d’étude et de recherche en microscopie électronique” of the University of Corsica (Corte, France).
3 Results
Observation of a large number of transverse and longitudinal sections of the spermatozoon of P. truncatus allowed us to distinguish four regions with distinctive ultrastructural characters.
3.1 Region I
Region I (Figs. 1–11 and 26I), corresponding to the anterior part of the spermatozoon, is characterized by the presence of two axonemes, an external ornamentation of the plasma membrane in entire length, spines-like bodies, a cytoplasmic expansion and numerous cortical microtubules. Cross sections of the anterior extremity of the spermatozoon show only cortical microtubules and the external ornamentation of the plasma membrane (Figs. 1-2). The first axoneme soon appears (Fig. 3), then the second (Fig. 4). In this part of the spermatozoon, number of cortical microtubules reaches its maximum with 41 (Fig. 5). However, transverse and longitudinal sections in this region show presence of an expansion of the cytoplasm (Figs. 5-6) and spinelike bodies interspersed with the membranar ornamentation (Figs. 6-7 and 9). External ornamentation of the cell membrane also lines the periphery of the sperm in the entire length of this region I (Figs. 1-11). Only two attachment zones are observed in cross sections of the spermatozoon (Figs. 8–11 and 26I).

Region I of the spermatozoon of Pleurogonius truncatus. Scale bar = 200 nm. (1-2) Cross sections of the anterior extremity of the spermatozoon showing only cortical microtubules (Cm) and the external ornamentation of the plasma membrane (Eo). (3) Cross section in the anterior part of the spermatozoon with the apparition of the first axonemal anterior extremity (Aae1), cortical microtubules (Cm) and the external ornamentation of the plasma membrane (Eo). (4) Cross section in the anterior part of the spermatozoon showing the first axoneme (Ax1), the second axonemal anterior extremity (Aae2), cortical microtubules (Cm) and the external ornamentation of the plasma membrane (Eo). (5) Cross section of the spermatozoon the two axonemes (Ax1 and Ax2), the external ornamentation of the plasma membrane (Eo), an expansion of the cytoplasm (Ce) and 41 cortical microtubules surrounding the axonemes. (6) Cross section of the spermatozoon showing the simultaneous presence of the two axonemes, a spinelike body (Sb), the external ornamentation of the plasma membrane (Eo), the expansion of the cytoplasm and 39 cortical microtubules surrounding the axonemes. (7) Longitudinal section of the spermatozoon with spinelike bodies (Sb) and the external ornamentation of the plasma membrane (Eo). (8) Cross section of the spermatozoon with two axonemes, the external ornamentation of the plasma membrane (Eo) and cortical microtubules (Cm). (9) Cross section showing two attachment zones (arrowheads), spinelike body, the external ornamentation of the plasma membrane and cortical microtubules. (10-11) Cross sections with the two axonemes, two attachment zones (arrowheads), a reduction of the external ornamentation of the plasma membrane and cortical microtubules.
3.2 Region II
Region II (Figs. 12–17 and 26II) is characterized by the presence of the two axonemes and the cortical microtubules. The external ornamentation of the plasma membrane disappears in this region. At the beginning of this region, cortical microtubules form a complete layer of 29 units around the cytoplasmic membrane (Fig. 12). After, they are disposed progressively in two reduced bundles. From the anterior to the posterior part of this region II, the number of cortical microtubules decreased from 29 (Fig. 12) to 27 (Fig. 13), to 22 (Fig. 14), to 19 (Fig. 15), to 9 (Fig. 16) and to 7 (Fig. 17). In this region two (Fig. 13) and four attachment zones are observed (Fig. 14–16). Cross sections in this region show also abundant glycogen granules revealed by the method of Thiéry (Figs. 25 and 26II).
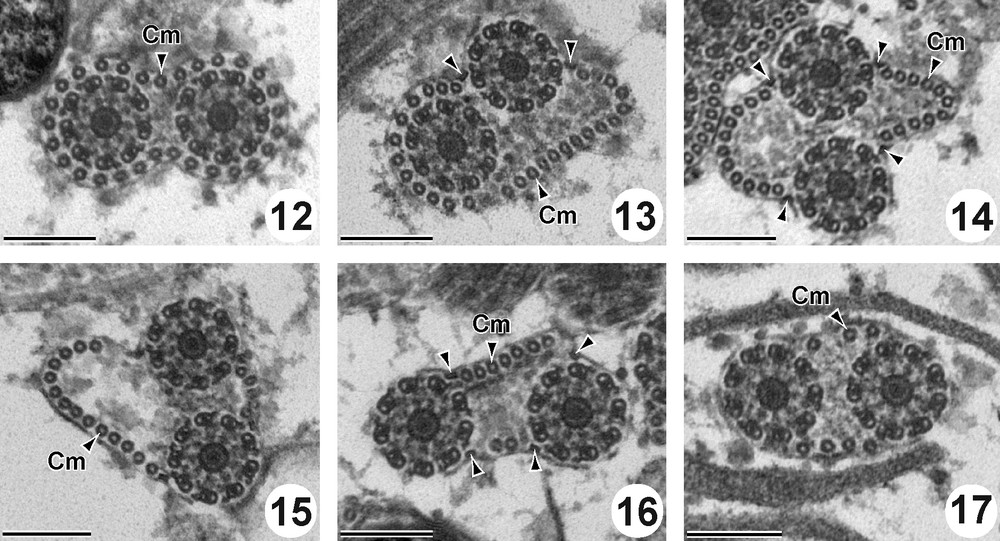
Cross sections in the Region II of the mature spermatozoon of P. truncatus. Scale bar = 200 nm. (12) Anterior part of the region II showing the two axonemes and cortical microtubules forming a complete layer of 29 units around the cytoplasmic membrane. (13-17) Two axonemes associated with two sets of cortical microtubules decreasing from 27 (Fig. 13), to 22 (Fig. 14), to 19 (Fig. 15), to 9 (Fig. 16) and to 7 (Fig. 17) and four attachment zones (arrowheads).

TEM photomicrograph of mature spermatozoa of P. truncatus showing granules of glycogen (G) revealed by the test of Thiéry. Scale = 1 μm.
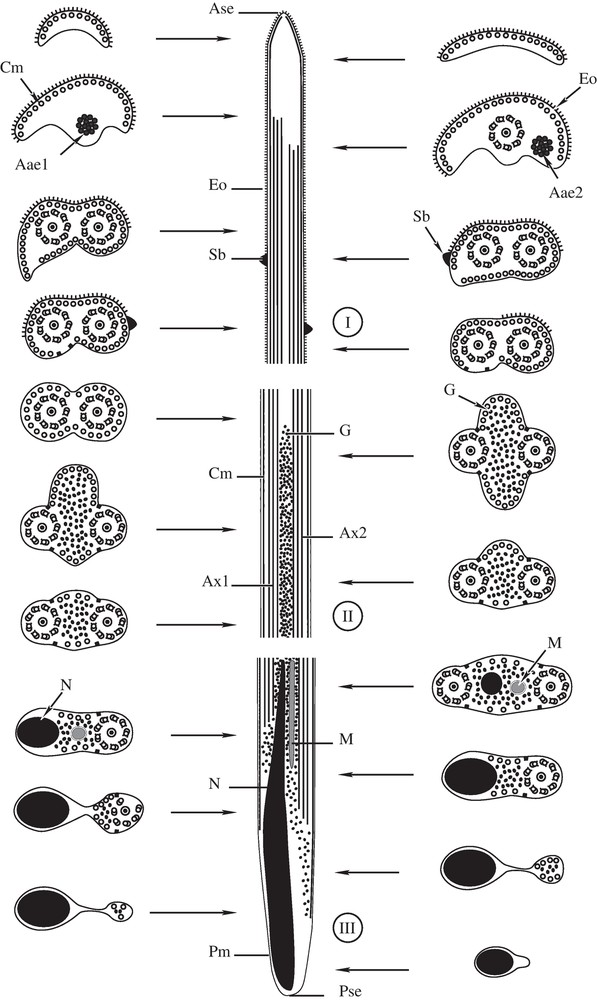
(I-III). Diagram showing the ultrastructural organization of the mature spermatozoon of P. truncatus, with indication of the three regions (I-III). Ase = anterior spermatozoon extremity, Aae1 = First axonemal anterior extremity; Aae2 = Second axonemal anterior extremity, Ax1 = axoneme 1, Ax2 = axoneme 2, Cm = cortical microtubules, Eo = external ornamentation of the plasma membrane, G = granule of glycogen, M = mitochondrion, N = nucleus, Pm = plasma membrane, Pse = posterior spermatozoon extremity, and Sb = spinelike body.
3.3 Region III
Region III (Figs. 18–24 and 26III). This region corresponds to the posterior part of the spermatozoon. In addition to the structures observed in the posterior part of the region II, this region is characterized by the simultaneous presence of the nucleus and mitochondrion in this anterior part (Figs. 18 and 26III). In this region, we observed the disappearance of the first axoneme (Fig. 19). The volume of the nucleus increases progressively and reaches its maximum in the middle part of this region III (Figs. 20-21). Then we observed successively the disappearance of the second axoneme (Figs. 22–23) and the cortical microtubules (Fig. 24). So the posterior extremity of the spermatozoon of P. truncatus is characterized by the presence of only the reduced nucleus (Fig. 24).

Cross sections in the Region III of the mature spermatozoon of P. truncatus. Scale bar = 200 nm. (18) Anterior part of the Region III with the simultaneous presence of two axonemes (Ax1 and Ax2), two reduced sets of cortical microtubules (Cm), four attachment zones (arrowheads), nucleus (N) and mitochondrion (M). (19) Simultaneous presence of nucleus (N) and mitochondrion (M), two attachment zones (arrowheads), two reduced sets of cortical microtubules and disappearance of the first axoneme (Ax1). (20) Section of the spermatozoon with only the second axoneme (Ax2), nucleus, two reduced sets of cortical microtubules and two attachment zones. (21) Great section of the nucleus (N), two reduced sets of cortical microtubules and the second axonemal posterior extremity (Pae2). (22-23) Nucleus (N), cortical microtubules (Cm) and two attachment zones. (24) Posterior extremity of the spermatozoon with only nucleus (N).
4 Discussion
Today, we lack data on the ultrastructure of the male gamete of the species of Pronocephaloidea. In this superfamily, there are only two studies, one in N. neyrai [10] and another in C. albus [11]. In this article, we have presented data pertaining to a third species of Pronocephaloidea: P. truncatus. The ultrastructure of the spermatozoon of P. truncatus exhibit the general pattern described in all the digenea. They present 2 axonemes with the 9 + “1” pattern typical of Trepaxonemata [13], mitochondrion, nucleus and parallel cortical microtubules described in all the digenea [14–24]. Spinelike bodies, cytoplasmic expansion and external ornamentation of the plasma membrane described in most of the digenea [10,11,16,18,21,25–30] are also observed in the spermatozoon of P. truncatus. The main particularity of the spermatozoon of P. truncatus is the ultrastructure of its anterior extremity. Most cross sections in this region show only the presence of cortical microtubules and the external ornamentation of the plasma membrane. In this case, the external ornamentation of the plasma membrane reaches all the anterior spermatozoon extremity. To our knowledge, such an anterior part of spermatozoon has never been described in digeneans. However, the anterior spermatozoon extremity of P. truncatus presents some similarities with than of C. albus, the only species studied now in Pronocephalidae (Table 1). Another similarity of the anterior region of spermatozoon between P. truncatus and C. albus is the coincidence of the maximum number of cortical microtubules with the lateral cytoplasmic expansion. The main difference between spermatozoa of P. truncatus and C. albus is the absence of the apical electron-dense materiel in P. truncatus. Ultrastructure of the anterior spermatozoon extremity of N. neyrai, another Pronocephaloidea, is very different of P. truncatus. In its anterior extremity, the spermatozoon of N. neyrai exhibits only one axoneme and lacks cortical microtubules and external ornamentation of the plasma membrane described in Pronocephalidae (Table 1). Today, presence of mitochondrion in the anterior part of the spermatozoon and absence of the cytoplasmic expansion are described in Pronocephaloidea only in N. neyrai (Ndiaye et al. [10]).
Comparison of few characters of Pronocephaloidean spermatozoa.
| Order | Families and species | Ase | Adm | Ce | Eo | Sb | M | Pse | References |
| Pronocephaloidea | Notocotylidae | ||||||||
| Notocotylus neyrai | 1 Ax | - | - | + | + | 2 | Axd | [10] | |
| Pronocephalidae | |||||||||
| Cricocephalus albus | C | + | + | + | + | 1 | N | [11] | |
| Pleurogonius truncatus | C | - | + | +* | + | 1 | N | Present study |
The posterior spermatozoon extremity with only nucleus, described in Pronocephalidae for first time in C. albus [11], is confirmed by the present study in P. truncatus. Like this, we proposed that the posterior end of spermatozoon of Pronocephalidae is characterized by the presence of only nucleus. It corresponds to the type 2 or Fasciolidean type described by Quilichini et al. [28] in Digeneans. According to these authors, this type is characterized by the absence of cortical microtubules, and the sequence: posterior extremity of the second axoneme then the posterior extremity of the nucleus. In Pronocephalidae we observe the same succession in C. albus [11] but in the case of P. truncatus (present study) the second axoneme disappears before the cortical microtubules. However, in N. neyrai [10] the posterior extremity of the spermatozoon corresponds to the type 3 or cryptogonimidean type defined by Quilichini et al. [28], characterized by the absence of cortical microtubules and the sequence posterior extremity of the nucleus then the posterior extremity of the second axoneme. All this confirm that the ultrastructure of the extremities of the spermatozoon is an efficient tool of phylogeny in the Platyhelminthes [4–6,8,28,31–34].
Attachment zones indicating the fusion of the two flagella with the median cytoplasmic process during the spermiogenesis are observed. We have observed two attachment zones both in the anterior and the posterior parts of the spermatozoon and four attachment zones between them associated with axonemes. These structures have been described by most authors in the spermatozoon of the digeneans [11,14,16,20,22,35–37].
To our knowledge, one or two or three mitochondria has been described in the spermatozoon of the digeneans [24]. In Pronocephalidae, we have described only one mitochondrion situated in the posterior part of the spermatozoon associated with the nucleus respectively in C. albus [11] and in P. truncatus (the present study). But in N. neyrai (Notocotylidae) we described a second mitochondrion in the anterior part of the spermatozoon [10]. So, we think that the number of mitochondrion in the spermatozoon is another interesting character of phylogeny within the Pronocephaloidea.
Disclosure of interest
The authors declare that they have no conflicts of interest concerning this article.
Acknowledgements
Authors wish to thank the fishermen of Ouakam (Dakar–Sénégal) for providing the specimens studied.


