1 Introduction
Hypoxic-ischemic (HI) brain damage is a clinical syndrome with high mortality and morbidity [1], and the survivors have suffered widely mental and physical disabilities [2]. However, there are no effective treatments available for HI brain damage at present.
Mesenchymal stem cells (MSCs), which are easily obtained from many tissues, have aroused great interest in regenerative medicine because of their multi-directional differentiation potential and hypo-immunogenicity properties [3]. In recent years, MSCs have showed a significant neuroprotective effects in experimental models of HI brain damage [4–6]. However, the main problem is that the low number of active cells reaching the target tissues after transplantation blocks its potential therapeutic effects [7,8].
Previous studies suggested that delivering the desired genes into the cells may overcome some limitations of cells and facilitate therapeutic efficiency after cell transplantation [9,10]. Hypoxia inducible factor-1 alpha (HIF-1α) is one of the most important regulators in response to oxygen levels, and the stabilization of HIF-1α in cells may promote activation and migration [11–13].
In the present study, we intended to overexpress HIF-1α in MSCs by recombinant lentivirus and investigate the issue whether HIF-1α overexpression could promote the therapeutic efficiency of MSCs.
2 Materials and methods
2.1 Animals
All procedures related to the use of animals complied with the guide published by NIH [14]. Adult male Sprague-Dawley rats (80–100 g) were purchased from the Animal Center of Zhejiang Chinese Medical University, Hangzhou, China (Laboratory Animal Certificate: scxk 2008-0016).
2.2 Generation, culture and pretreatment of MSCs
MSCs were obtained from rat femoral and tibia bone marrow as previously described [15]. Briefly, muscles, connective tissue and epiphyses were removed from femur and tibia. Marrow was harvested by 1.0 mL syringe with cell medium that consist of Dulbecco's modified Eagle's medium/Ham's F-12 (DMEM/F-12; Invitrogen, Grand Island, NY, USA), 10% fetal bovine serum (FBS; Life Technologies, Carlsbad, CA, USA) and 1% penicillin-streptomycin (HyClone Laboratories, Inc., South Logan, Utah, USA). Cells were then centrifuged for 10 min at 1000 rpm, re-suspended in the cell medium and cultured at 37 °C, 5% CO2. After 24 h, all non-adherent cells were removed by medium exchange; the fresh medium was subsequently replaced every 2 days. The adherent cells were trypsinized at 80% confluence, and passaged at 1:2. MSCs from passage 3 were used in the subsequent experiments.
MSCs had received a hypoxia pretreatment during 24 h to mimic the microenvironment in the damaged area in order to activate the endogenous gene expression, which is the natural effect of cells in response to hypoxia. Briefly, the cells were placed in a hypoxia incubator and balanced with a gas mixture (1% O2, 5% CO2, 94% N2) for 10 min at a flow rate of 1 L/min. Then, the hypoxia incubator was sealed to maintain a hypoxic environment before being put into a cell incubator under 5% CO2 at 37 °C.
2.3 Lentivirus-mediated gene transfer and experimental groups of cells
MSCs with HIF-1α overexpression were constructed by a lentivirus system following the manufacturer's instructions (Invitrogen, Carlsbad, CA, USA). The cells were infected with a lentivirus encoding either GFP alone or both GFP and HIF-1α. Then the MSCs were assigned into three experiment groups: HIF-1α-MSCs (transfection with HIF-1α and GFP), GFP-MSCs (transfection with GFP), NM-MSCs (undisturbed normal MSCs).
The transfection efficiency of the cells was detected by fluorescence microscopy analysis after transfecting for 48 h. The protein expression level of HIF-1α in MSCs was detected by Western blotting.
2.4 Western blotting
The expression level of HIF-1α was evaluated by western blotting after hypoxia pretreatment. Equal amounts of protein from MSCs were separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Boston, MA). After being blocked with 5% nonfat dry milk for 2 h, the membranes were incubated with rabbit polyclonal antibodies against either HIF-1α (1:1000 dilution, Abcam Technology, Cambridge, MA, USA) or GAPDH (1:2000 dilution, Abcam Technology, Cambridge, MA, USA) overnight at 4 °C. The membranes were then incubated with a second antibody (anti-rabbit IgG HRP, 1:3000; Abcam Technology, Cambridge, MA, USA) and ECL detection systems (Thermo Fisher Scientific, Waltham, MA, USA) were used for detection, GAPDH was used as a loading control.
2.5 MTT assay
An assay with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was performed to determine the influence of HIF-1α overexpression on MSC viability. Briefly, pretreated MSCs were seeded in 96-well plates at a density of 2000 cells per well, and cultured for 48 h, and NM-MSCs were used as a control. 10 μL (5 g/L in PBS) of MTT (Sigma Chemical Co., St. Louis, MO, USA) were added to each well and incubated at 37 °C for 4 h. The supernatants were carefully discarded and 150 μL of dimethyl sulfoxide (Sigma Chemical Co., St. Louis, MO, USA) was added. Absorbance was measured at 490 nm by microplate reader (Bio-RAD Model 680, Cal, USA).
2.6 CM-DiI labelling
In order to make it easily observable, MSCs were stained with chloromethylbenzamido dialkylcarbocyanine (CM-DiI, Molecular Probes, USA) at the dose of 1 μg CM-DiI per million cells, and washed twice after incubation at 37 °C for 20 min. Labeled MSCs were used in the following experiments.
2.7 Migration assay
A cell migration assay was performed in Transwell chambers (pore size: 8 μm, polycarbonate membrane; Corning Incorporated, NY, USA) to evaluate the migratory capacity of MSCs. Transwell chambers were put into six well plates. CM-DiI labeled cells were harvested in a serum-free medium; the density was adjusted to 2 × 105 cells/ml. 200 μL of cell suspension were plated in the upper chamber and 500 μL of a fresh medium containing 10% FBS were added to the lower chamber. After incubation at 37 °C under 5% CO2 for 24 h, the cells on upper membrane layer were gently wiped away using a cotton swab and the cells migrating to the lower membrane were fixed with 4% paraformaldehyde. The number of cells in the lower side of the membranes was counted microscopically.
2.8 Hypoxic-ischemic model and experimental groups of rats
As HI model was established as in the previous method of Rice et al. [16]. The rats were anesthetized with a 10% chloral hydrate solution (0.3 mL/100 g), and the left carotid artery was isolated and ligated doubly. After 2–3 h, the waking rats were put into a transparent container which was ventilated with a constant flow of mixed gas containing 8% oxygen and 92% nitrogen for 2 h.
The animals were randomly assigned into four experimental groups after HI treatment: sham group, HI-vehicle group, HI-GFP-MSC group, HI-HIF-1α-MSC group. For the rats in the sham group, the carotid artery was isolated but not ligated, and no hypoxia exposure was performed. The rats in the HI-GFP-MSC group and the HI-HIF-1α-MSC group had received 5 × 105 MSCs re-suspended in 0.5 mL of a physiological saline solution by intravenous injection in the tail 24 h after HI treatment, and the other rats received the same volume of physiological saline solution.
2.9 Morris water maze test
Rats (n = 6 per group) have been submitted to the Morris water maze test [17] on day 14 after HI to evaluate their spatial learning and memory abilities. The maze consisted of a black circular tank (160 cm diameter × 50 cm height) filled with water (temperature: 22–24 °C). An automatic tracking system (San Diego Instruments, San Diego, CA, USA) was used to record the swimming path and time. Each rat was tested for five training days and one test day. In the task, the rat was put in every quadrant of the pool sequentially, and swam for 60 s to locate the submerged platform; if it failed, the experimenter would guide it to the platform. Then the rat was allowed to remain on the platform for 10 s. On the last day, the platform was removed before the test, and the swimming time in the former platform quadrant was recorded.
2.10 Tissue preparation and histological examination
Rats brains were obtained on days 7, 14 and 21 after HI (each time point n = 6 per group), and each hippocampus was embedded in paraffin. The paraffin sections (4 μm thick) were prepared for detection of the migration of MSCs and histological examination in the hippocampus. To measure the migrated cells’ number, the sections were observed with a fluorescence microscope to count the CM-DiI positive cells. The histological examination was determined by hematoxylin and eosin (HE) staining.
2.11 Statistical analysis
The data obtained were presented as mean ± standard deviation (SD). Differences between groups were evaluate by analysis of variance for multiple comparisons using SPSS 19.0. A value of P < 0.05 was considered to be statistically significant.
3 Results
3.1 Overexpression of HIF-1α in MSCs
After transfection for two days, GFP expression observed under the fluorescence microscope reached up to 90% of the cultured MSCs (Fig. 1A). In the meantime, western blotting was performed to investigate the expression of HIF-1α in MSCs after hypoxia pretreatment. In the present study, the expression of HIF-1α in HIF-1α-MSCs was approximately two times that in NM-MSCs and GFP-MSCs, while the expression level in GFP-MSCs was in accordance with NM-MSCs (Fig. 1B and C). The results indicated that HIF-1α overexpressed MSCs were successfully constructed by a recombinant lentivirus system.
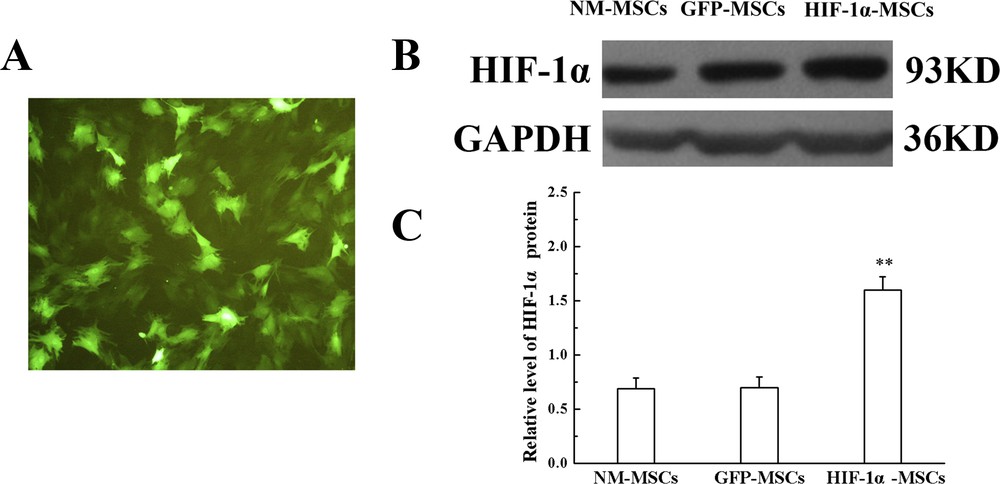
Transfection efficiency of MSCs. A. The level of GFP expressed in MSCs was observed by fluorescence microscopy (magnification × 100). B. Protein level of HIF-1α in different groups; GAPDH was used as an inner control. C. Quantification of HIF-1α protein expression showed in B. **P < 0.01 versus the GFP-MSCs group.
3.2 HIF-1α overexpression improved the viability of MSCs
MSC viability was assessed by an MTT assay to determine the effect of HIF-1α overexpression. As shown by our results, HIF-1α overexpression immensely improved the viability of MSCs, while there was no significant difference between NM-MSC and GFP-MSC groups (Fig. 2A). The result implied that MSCs with overexpression of HIF-1α had better viability.
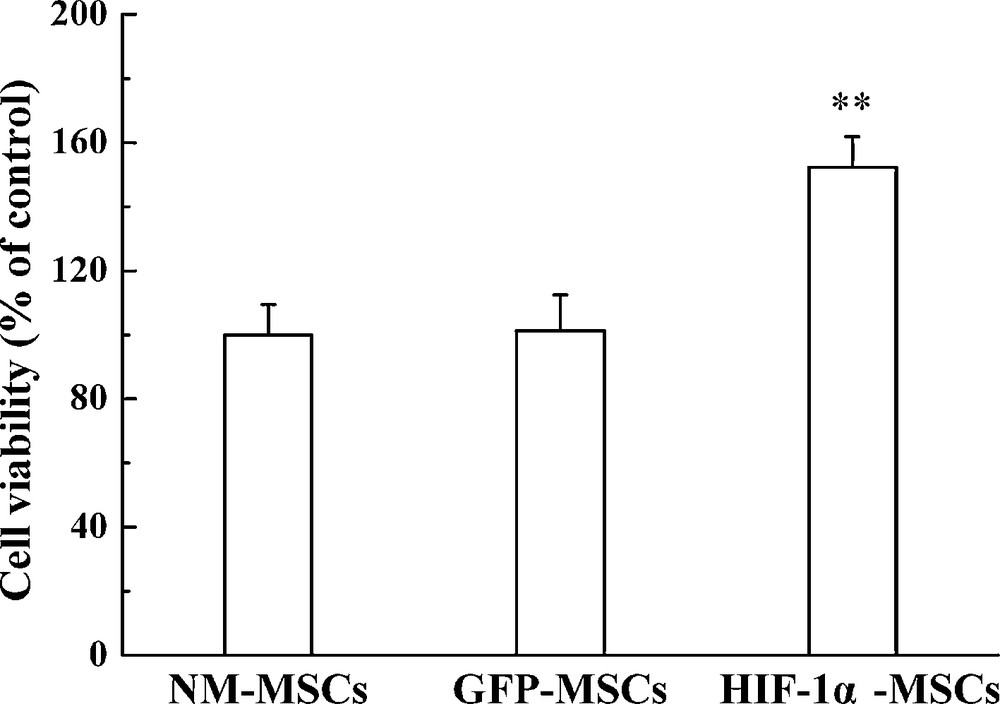
MTT assay used to quantify the MSCs viability in different group. **P < 0.01 versus the GFP-MSCs group.
3.3 HIF-1α overexpression enhanced migration of MSCs both in vitro and in vivo
The migration of MSCs was analyzed both in in vitro and in vivo experiments. To observe the migration of MSCs directly, the cells were labeled with CM-DiI, a fluorescence marker. The results showed that more than 95% of MSCs were CM-DiI positive after having been labeled (Fig. 3A).
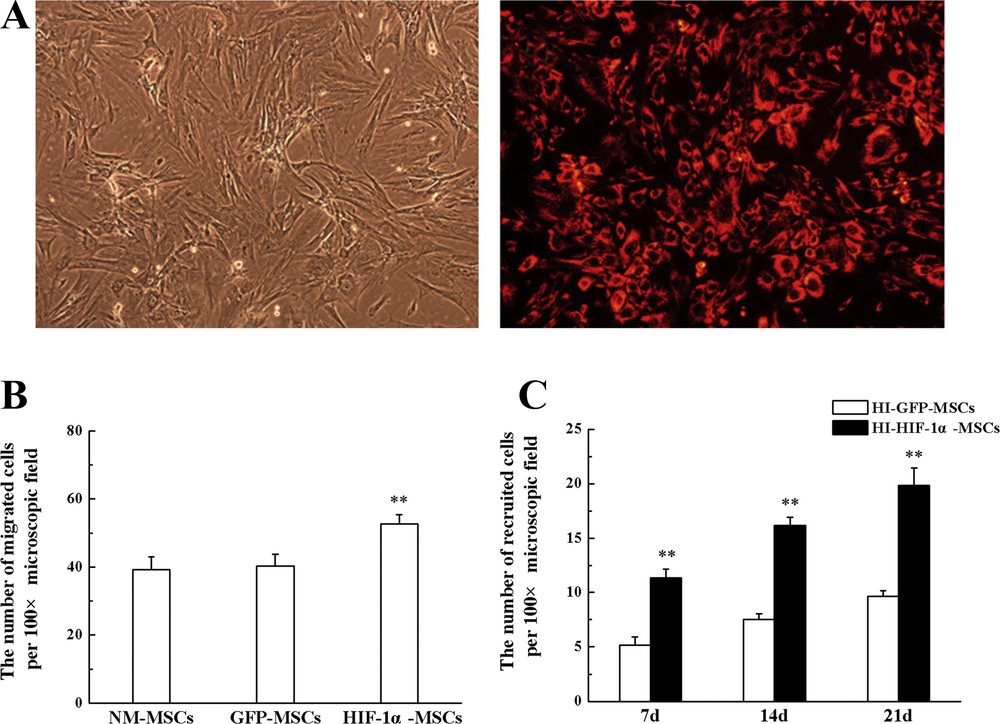
MSCs migration assay in vitro and in vivo experiments. A. The phase-contrast and fluorescent image of CM-DiI labeled MSCs (magnification: × 100). B. Transwell migration assay, performed in vitro experiments. C. Transplanted MSCs homing to the hippocampus were counted at different time points. **P < 0.01 versus the GFP-MSCs group.
According to the Transwell assay, the number of cells migrating to the lower compartment in HIF-1α-MSC group was more than that in NM-MSC and GFP-MSC groups after hypoxia pretreatment, while no significant difference was found between NM-MSC and GFP-MSC groups after culturing for 24 h (Fig. 3B).
One day after HI, rats received an intravenous transplantation of MSCs, and the presence of migrated MSCs in the hippocampus after transplantation was investigated by observing CM-DiI positive cells. CM-DiI positive cells were clearly observed in the hippocampus on day 7 after HI. And from day 7 to day 21 subsequent to HI, the number of CM-DiI positive cells in the hippocampus increased gradually in a time-dependent manner. A remarkable increase in the recruitment of HIF-1α transduced MSCs was observed in the hippocampus at every time point compared with GFP transduced MSCs (Fig. 3C). Statistical analysis showed that HIF-1α overexpression improved the migration capacity of MSCs toward the sites of injury.
The quantitation of migrated cells demonstrated significant increases in MSCs motility in HIF-1α overexpressed MSCs both in in vitro and in vivo experiments in comparison to GFP infected cells.
3.4 HIF-1α overexpression strengthen the therapeutic efficiency of MSCs on HI rats
In order to investigate the therapeutic effect of HIF-1α transduced MSCs, the Morris water maze test and HE staining in the hippocampus were performed on HI rats. On day 14 after HI, the spatial performance of four groups was evaluated by the Morris water maze test. In the spatial probe test, spatial learning ability was significantly affected by HI, as HI-treated rats spent less time in the target quadrant compared to the sham group. The results suggested that the rats suffered from cognitive dysfunction due to HI treatment. There was a significant increase of time in the target quadrant in both MSC-transplanted groups compared with HI-vehicle group, but a more increase in the amount of time have been found in HI-HIF-1α-MSC group (Fig. 4A). These results indicated that the HI-induced rats achieved partial remission on memorial and cognitive functions after MSCs transplantation, while HIF-1α overexpression could promote this effect.
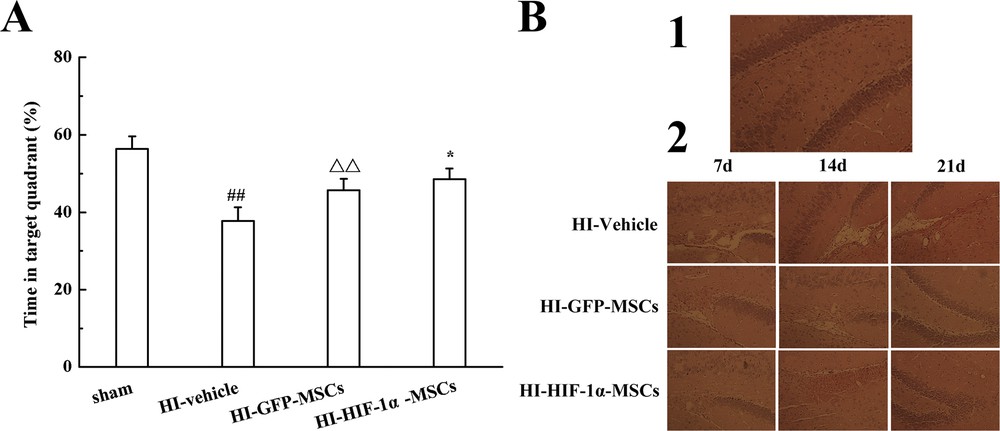
The therapeutic efficiency of HIF-1α overexpressed MSCs in HI rats. A. Percentage of swimming time in the target quadrant in the Morris water maze. B. HE staining in different groups of the hippocampus. Representative images in the sham group (1), the pathological changes in the HI-Vehicle, HI-GFP-MSCs and HI-HIF-1α-MSCs groups at different time points (2). ##P < 0.01 versus the sham group, ΔΔP < 0.01 versus the HI-Vehicle group, *P < 0.05 versus the HI-GFP-MSCs group.
Some visualized pathological changes were found in HE staining. The data supported that the morphological structures of the hippocampus in the sham group were normal, with uniform dyeing (Fig. 4B1). In contrast with the sham group, the pathological changes in the hippocampus in the HI-vehicle group revealed serious injury, the cells were disordered, and cavitation was visible, suggesting that the HI model had been successfully established. Meanwhile, the rats in HI-GFP-MSC and HI-HIF-1α-MSC groups showed similar but slight pathological changes at every time point after having been subjected to HI, and the changes in the MSCs with HIF-1α transfection group were less marked than those of the GFP control group (Fig. 4B2). The results illustrated that the transplantation of MSCs ameliorated pathological changes after HI, while HIF-1α overexpression could enhance therapeutic efficiency.
4 Discussion
In recent years, abundant researches show that MSCs transplantation has been considered a promising strategy in HI-induced brain-damaged treatment [4,18,19]. However, the insufficient viability as well as the low number of cells transplanted to the target tissues limits the potential therapeutic effects [7,8]. Recent evidences have indicated that HIF-1α is important in cell viability, proliferation and migration following HI injury [12,13]. In the present study, we therefore investigated the therapeutic effect of HIF-1α overexpressed MSCs on HI brain damage. Firstly, overexpression was achieved using a recombinant lentiviral, and a robust overexpression of HIF-1α at the protein level has been seen. Additionally, the functions of HIF-1α overexpression in MSCs showed that HIF-1α overexpression resulted in increased viability and migration of MSCs. Finally, using a HI model, we found that HIF-1α overexpressed MSCs had a stronger therapeutic efficiency on HI brain-damaged treatment by mitigating the injury on behavioral and histological changes evoked by HI insults, accompanied with more MSCs migrating to the damaged cerebral area.
The hypoxia-inducible factor-1 (HIF-1) is recognized as the master regulator of hypoxia response. It is a heterodimeric transcription factor composed of an inducible HIF-1α subunit and a HIF-1β subunit, and HIF-1α is a crucial mediator of the cellular response to hypoxia in contrast to HIF-1β, implying that HIF-1α is a key mediator of the beneficial effect in a low oxygen environment [20]. In this study, just as expected, we have seen the expression of HIF-1α after hypoxia pretreatment, while overexpression increased the expression.
The present study showed that overexpression of HIF-1α enhanced MSCs viability in vitro, which was consistent with a previous study [21]. Intriguingly, the results also showed in our present study that HIF-1α overexpression could further promote the viability of MSCs in vitro in comparison to the effect of HIF-1α induced by hypoxia pretreatment, indicating that the expression level of endogenous HIF-1α, which achieved during hypoxia, does not reach the maximum required for HIF-1α transcription activities, and transfection with exogenous HIF-1α improves the transcription level.
Cerebral ischemia and hypoxia will result in cognitive dysfunction [22,23] as well as pathological changes [24], especially the changes in the hippocampus [25], which showed in the Morris water maze test and HE staining in our study. Transplantation of MSCs after HI contributed to an improved function recovery of neurological deficits and reduced the degree of pathological changes [18,19,26]. Meanwhile, it was observed that HIF-1α overexpression led to a better efficiency of MSC transplantation. This result is not in keeping with studies by Lopez et al. [27], which showed that overexpression of HIF-1α did not have protective actions upon hypoxia-mediated neuronal death. However, enhanced therapeutic efficiency of cells on brain damage through upregulation of HIF-1α also been found in other groups [28,29]. These controversial results may be explained by the different damage degree (HI vs. mild hypoxia).
We also investigated the recruitment of MSCs in the hippocampus in time course experiments; the present study results together with that from previous study [30] demonstrated a number of MSCs in the hippocampus increasing continuously three weeks after transplantation. Consistently with in vitro experiments, HIF-1α overexpression also improved MSCs migration to the injured area. The upregulation of HIF-1α increased the migration of MSCs, as well as it enhanced the therapeutic efficiency of MSCs, which was also found by other groups [31,32]. Thus, enhancing the number of MSCs to the site of injury may improve the therapeutic efficiency of MSCs transplantation. In other words, the improvement of the therapeutic efficiency through overexpression of the HIF-1α of MSCs could be due to increased cell migration to the damaged area.
The limitation of this study is that the underlying associated mechanisms about HIF-1α promoting the directional migration of MSCs to the cerebral HI area need to be confirmed, which we will investigate in further studies.
In conclusion, this study demonstrated that HIF-1α overexpression could increase the therapeutic efficiency MSCs in HI, which will correlate well with the promotion cells’ directional migration to the cerebral HI area. We propose that transplantation of MSCs with HIF-1α overexpression is an attractive therapeutic option to treat HI-induced brain injury in the future.
Acknowledgments
This work was financially supported by National Natural Science Foundation of China (Grant No. 81270566, Grant No. 81500114).


