1 Introduction
The TeO2-based glasses form a highly interesting field of recent studies aiming at basic scientific goals as well as research for new promising materials for non-linear optics. However, whereas the exceptional dielectric and optical properties of those materials are usually associated with the E-pairs of Te(IV), the nature of their mechanical and thermal properties remains an open question. Evidently, such properties must be related to the presence of the Te–eqOax–Te bridges, which always exist in the condensed TeO2, and in tellurite structures, having complex TenOm anions 〚1〛. Those bridges, as a rule, are essentially asymmetric, since the equatorial Te–O bonds are mainly covalent, and the axial ones are mainly electrostatic 〚2〛.
Thus, it can be thought that the properties in question can be controlled by the polymerisation (homogenisation) of the Te–O–Te bridges. In particular, this can be done by introducing the fluorine atoms into the coordination spheres of tellurium. In the cases of the Te–O–F system, the atoms of fluorine (being more electronegative than oxygens) would accept some electrons of tellurium, thus allowing each Te atom to form the two simple Te–O bonds 〚3〛, which can polymerise into the Te–O chains, having Te–O–Te bridges similar to Si–O–Si bridges. In those chains, the TeO2 fragments can be readily distinguished, like the SiO4 units can be distinguished in the SiO2 framework.
Thus, whereas the pure tellurium dioxide systems would have quasi-isolated TeO2 units that cannot polymerise 〚4〛, it appears that the existence of polymerised Te–O chains is intrinsic for Te–O–F systems. Such a sharp difference in the electronic structure and crystal chemistry of those objects must be reflected on their fundamental physical properties. At present, a number of works have been devoted to the investigations of tellurium–oxyfluoride mixed systems 〚5–12〛.
Our previous study of the TeO2–TeF4 system has clearly shown the influence of F atoms as a factor enlarging the glass forming domain and favouring the structural evolution from a three-dimensional (but weakly polymerised) network (TeO2) into a well-pronounced chain-like one (TeOF2) via a sheet-like framework (Te2O3F2) 〚3, 13〛. From these points of view, we are now investigating the TeO2–Tl2O–TlF system. Our previous study of the TeO2–Tl2O system clearly demonstrated that non-linear refractive indices of thallium tellurite glasses could be up to 100 times as large as those of SiO2 and that increasing the thallium content leads to the transformation of TeO4 disphenoids into isolated TeO3 trigonal pyramids and to the decrease of the number of Te–O–Te linkages 〚14〛. In this work, we have evidenced the glass-forming domain within the TeO2–TlF and Tl2Te3O7–TlF systems. The densities and the thermal behaviour of the glasses (glass transition and crystallisation temperatures) have been determined. A structural study of thallium fluorotellurite glasses has also been approached.
2 Experimental
Glassy samples were prepared by first melting at 800 °C for 20 min in sealed gold tubes and then air-quenching intimate mixtures of TlF (Aldrich, 99.9%) and TeO2 (for the study of the TeO2–TlF system) or TlF and Tl2Te3O7 (for the study of the Tl2Te3O7–TlF system). TeO2 was prepared by decomposition at 550 °C under flowing oxygen, of commercial H6TeO6 (Aldrich). Tl2Te3O7 was obtained by heating, at 350 °C for 18 h then at 250 °C for 24 h, in a gold crucible under pure flowing nitrogen, various mixtures of high purity Tl2CO3 (Aldrich, 99%) and TeO2. Glass-formation domains were determined by using X-ray diffraction (Guinier–De Wolff camera, Cu Kα radiation). Glass transition (Tg) and crystallisation (Tc) temperatures were measured by heat flux differential scanning calorimetry (Netsch STA 409). The powdered samples (≈ 30 mg) were introduced into non-sealed platinum crucibles and the DSC curves were recorded between 20 and 800 °C maximum, using a heating rate of 10 °C min–1, under pure nitrogen atmosphere. The densities of glassy samples were measured on finely ground powders by helium pycnometry (Accupyc 1330). A structural approach of the glasses was realised using Raman scattering. The Raman spectra were recorded in the 80–1200 cm–1 range using a Dilor spectrometer (XY model), equipped with a CCD detector and an Ar+ laser (514.5 nm exciting line) in a backscattering geometry. With such a detector, a good signal/noise ratio needed one or at most two scans (during 100 s). The samples were visually controlled through a microscope (× 100), which has enabled us to analyse a surface with diameter of about 2 μm. The diameter of the laser spot focused on the sample was about 1 μm. Reproducibility of the results thus obtained was a proof of identity and homogeneity of the samples. Measurements were made at low power (< 100 mW) of the excitation line, in order to avoid any damage of the samples. The spectral resolution was about 4.5 cm–1 at the exciting line.
3 Results and discussion
3.1 Formation and thermal behaviour of glasses
Under our experimental conditions, melting at 800 °C and then air quenching, large glassy domains have been evidenced within the TeO2–TlF (from 7 to 65 mol% of TlF) and Tl2Te3O7–TlF (from 0 to 75 mol% of TlF content) pseudo-binary systems (Fig. 1). With respect to the glassy domain observed within the TeO2–Tl2O system 〚15〛 under the same conditions of elaboration, 15 to 52 mol% of TlO0.5, it is clearly seen that addition of F atoms allows enlarging the glass-forming domain. Within the TeO2–TlF system, the glasses are all homogeneous and white; their density increases from 5.7 to 7.06 g cm–3 with increasing the TlF content. Within the Tl2Te3O7–TlF system, the glasses are also homogeneous. Their colour changes from yellow to white with increasing the TlF content; their density increases from 6.72 to 7.21 g cm–3 with increasing the TlF content. The evolutions within these two systems of the glass transition and crystallisation temperatures, as a function of the composition, are shown in Fig. 2. As a comparison, the evolution observed with TeO2-Tl2O glasses is also given.

Glass-forming domains within the TeO2–Tl2O, TeO2–TlF and Tl2Te3O7–TlF systems.
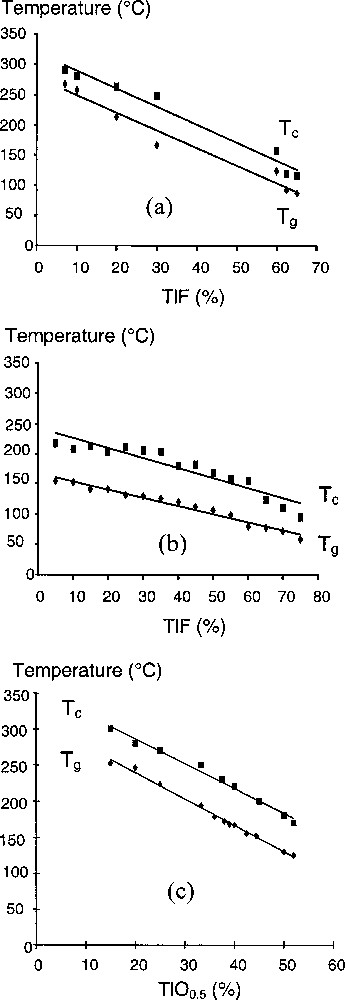
Evolutions with composition of the glass transition (Tg) and crystallisation (Tc) temperatures within the (a) TeO2–TlF, (b) Tl2Te3O7–TlF and (c) TeO2–Tl2O systems.
Within our two fluorotellurite systems, Tg and Tc temperatures continuously decrease with increasing the TlF content, as previously observed with increasing the TlO0.5 content. This decrease can be attributed to the thallium addition. It is interesting to note that the difference between Tc and Tg, which characterises the thermal stability of the glass, remains practically constant with increasing TlF content (for all the compositional range within the TeO2–TlF system and at least up to about 65 mol% of TlF within the Tl2Te3O7–TlF system). However, the thermal stability of TeO2–TlF glasses is slightly lower than that observed with thallium tellurite glasses. This can indicate that the substitution of O atoms by F ones would decrease the thermal stability of glassy samples. Above 60 mol% of TlF content, Tl2Te3O7–TlF glasses are characterised by a sharp decrease of their Tc temperature, whereas their Tg temperature decreases slowly, thus reducing their thermal stability. However, such glasses exhibit a thermal stability in the range 55–95 °C considerably superior to that of TeO2–TlF ones. A study of the crystallisation (especially the nature of the first phase that crystallises from glass) of such samples is now in progress using in-situ powder XRD. Preliminary results have confirmed the existence of the metastable γ-TeO2 polymorph 〚4,16〛 for TeO2-rich samples.
3.2 Structural aspects of the TeO2–TlF glasses
The structural aspects of our study were mainly considered by analysing the evolution of the Raman spectra of the x TeO2/(1 – x) TlF system (0.35 ≤ x ≤ 1), and comparing it with that of the x TeO2/(1 – x) TlO0.5 system. Raman spectra of the latter (Fig. 3b) show that with decreasing x, the solid TeO2 continuously evolves into the Tl2TeO3 system, in which all the TeO2 molecules become the isolated 〚TeO3〛2– orthoanions having the two principal vibrations: a stretching pulsation (near 725 cm–1) and a symmetric bonding of the TeO3 pyramids (≈ 300 cm–1). Another type of structural evolution can be proposed from the analysis of the Raman spectra of the x TeO2/(1 – x) TlF system. Actually, for 0.6 ≤ x ≤ 1, the Raman spectrum keeps its view typical for (pure) glassy TeO2. Beginning from x = 0.6, the quantity of glassy TeO2 sharply drops: the intense band near 450 cm–1 (indicating Te–O–Te bridges) disappears, and a strong band appears above 750 cm–1, which indicates the formation of isolated groups having short (essentially non-bridging) Te–O bonds; a very weak band around 300 cm–1 can be attributed to the bonding vibrations of O–Te–O angles.
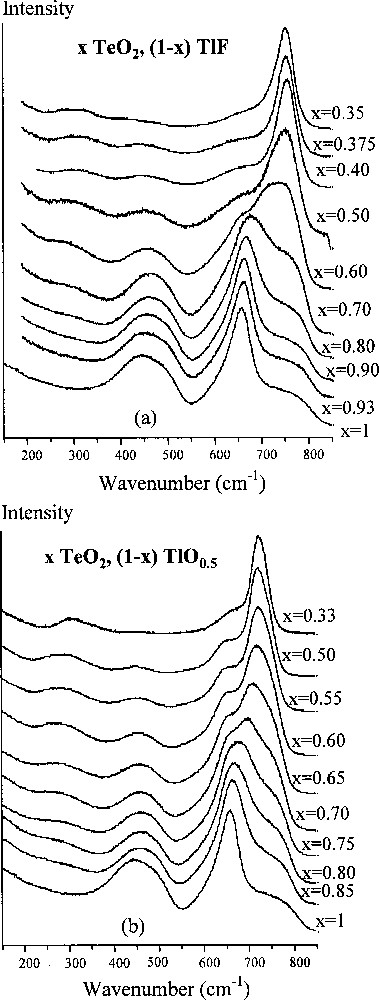
Raman spectra of (a) TeO2–TlF glasses and (b) TeO2–Tl2O glasses (for the latter system, the glassy domain has been extended from 0 mol% TlO0.5 up to 66.7 mol% TlO0.5 under the extreme conditions of first melting at 800 °C and then ice quenching).
Thus, no indication of the Te–O chains (i.e. Te–O–Te polymerisation) was observed in the Raman spectra. This fact could be explained by the presence of Tl+ cations. To our belief, for 0.6 ≤ x ≤ 1, the two phases, TlF and TeO2, coexist in the glasses independently. The mutual influence begins when x ≤ 0.6, and it results in a reaction of TeO2 molecules with fluorine atoms, thus forming isolated groups containing non-bridging Te–O bonds. The concrete structure of those groups will be the object of our further XRD study of the relevant crystalline phases.
Acknowledgements
One of us, C. Lasbrugnas is grateful to the ‘Conseil régional du Limousin’ for financial support.



