1 Introduction
When doped with rare-earth ions, fluoride glasses are well known materials for fibre lasers and fibre optical amplifiers, since their performances are much higher than for silica 〚1〛; for example, optical amplification has been demonstrated at wavelengths not attainable from silica fibre, namely at 1.3 μm, with praseodymium for the second telecommunication window. In the past decade, the need for low-cost and more compact optical devices has motivated the research on integrated systems. Several methods can be used to create these structures either by sol–gel synthesis, ionic exchange and vapour deposition techniques such as Chemical Vapour Deposition (CVD), Physical Vapour Deposition (PVD) 〚2–6〛. The efficient vapour deposition techniques yield not only the requisite purity and optical quality but also the means for making precise waveguide structures.
This paper describes the challenge for preparing rare-earth-doped fluoride glass planar and channel waveguides by the PVD technique.
2 Fluoride glass composition
A new family of fluoride glass has been discovered by J.-P. Miranday in Le Mans in 1979, in the PbF2–MF2–GaF3 systems with M = Mn2+, Zn2+ 〚7〛; the vitreous domain is quite large as shown in Fig. 1. These glasses show an original network where ZnF6 and GaF6 octahedra share corners forming chains with the large Pb2+ cations randomly distributed; the crystal chemistry is totally different from the ZrF4-based glasses with high coordination (7 and 8) and vitreous BeF2 built up from tetrahedra. Table 1 lists the standard compositions of zirconium fluoride glass (ZBLA) and gallium fluoride glass (PZG).
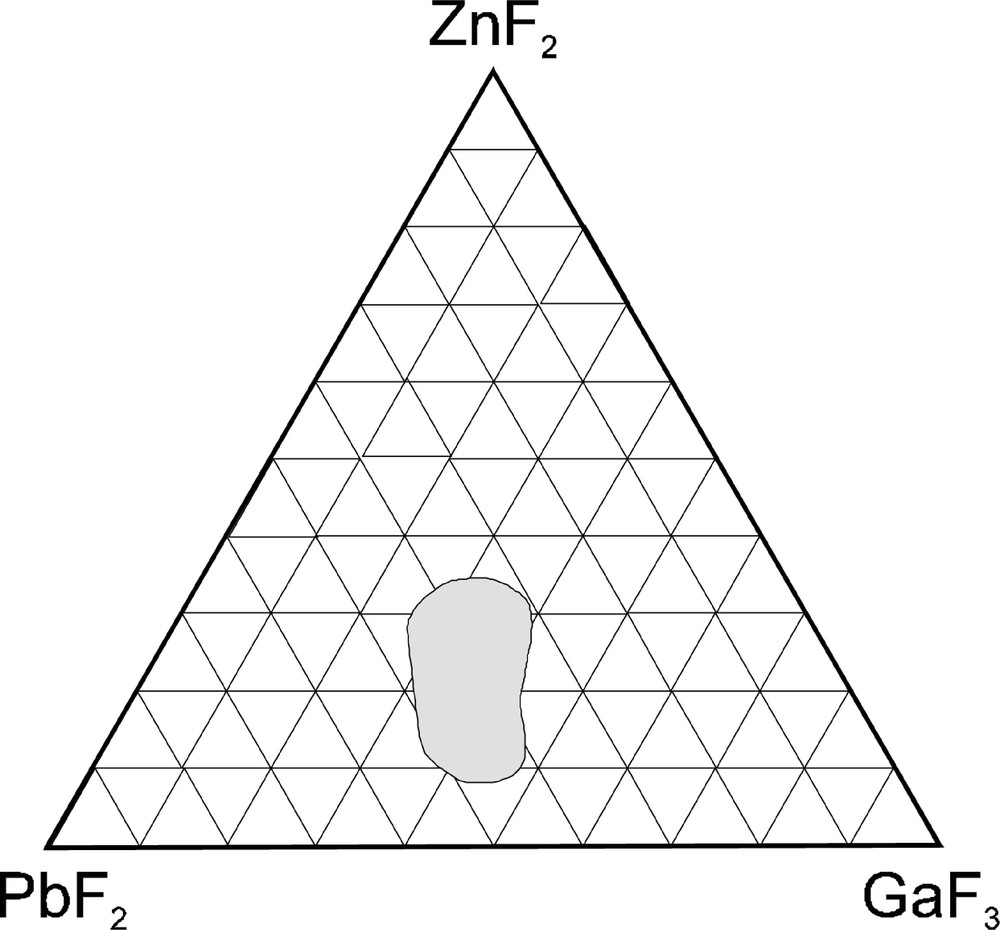
Vitreous domain in the PbF2–ZnF2–GaF3 system.
Examples of some optimised fluoride glass compositions (mol%).
| Zirconium fluoride glass (ZBLA) | ZrF4 | 57 | nD = 1.511 |
| BaF2 | 34 | ||
| LaF3 | 5 | ||
| AlF3 | 4 | ||
| Gallium fluoride glass (PZG36) | PbF2 | 36 | nD = 1.577 |
| ZnF2 | 24 | ||
| GaF3 | 35 | ||
| AlF3 | 2 | ||
| YF3 | 5 | ||
| Gallium fluoride glass (PZG45) | PbF2 | 45 | nD = 1.640 |
| ZnF2 | 20 | ||
| GaF3 | 30 | ||
| AlF3 | 2 | ||
| YF3 | 5 |
The phonon energy of the matrix is governed by the metal fluorine bond of the glass former, which gives rise to vibrational modes of much lower frequencies than the traditional glass as silicates, borates; for example, the Ga–F, Zr–F and Si–O elongation modes are located respectively at 560, 580 and 1100 cm–1. The phonon energy is actually a key parameter for optical properties of the glassy matrix since low values lead to higher transparency in the infrared and also provide active dopants with longer excited state lifetimes. Silica does not readily accept rare-earth ions as a dopant because of the low coordination number of the silica tetrahedron. In fluoride glasses, except BeF2, the rare-earth ions can occupy sites of high coordination by substitution to Zr4+ in ZBLAN and Pb2+ in PZG glasses; thus, the solubility is higher than in silica, up to a few mol% depending on the size of the rare earth.
3 Optical waveguide fabrication
3.1 Vapour pressure of fluorides
Physical vapour deposition needs congruent evaporation from a glassy melt. This criterion is highly restrictive for fluoride glasses, since most of them are composed of at least three metallic fluorides. In most cases, the volatilities of the individual fluorides are too different to enable a vapour phase whose composition corresponds to a multicomponent stable glass (Fig. 2). For example, the evaporation of ZBLA glass has failed because of the much higher volatility of ZrF4 compared with that of BaF2 〚8, 9〛. On the contrary, the vapour pressure of PbF2, GaF3 and ZnF2 are quite close. As a matter of fact, we have demonstrated the possibility of creating a ternary system in the vapour state by adjusting the composition of the starting melt, a mixture of the volatile fluorides PbF2, ZnF2 and GaF3 diluted in a low vapour pressure fluoride melt 〚5〛. The composition of the glass is quite different from that predicted on the basis of the equilibrium vapour pressures of the individual glass constituents, as shown in Table 2. This result indicates a high degree of association in the vapour; at least, the existence of PbGaF5 entities is highly probable 〚5〛.
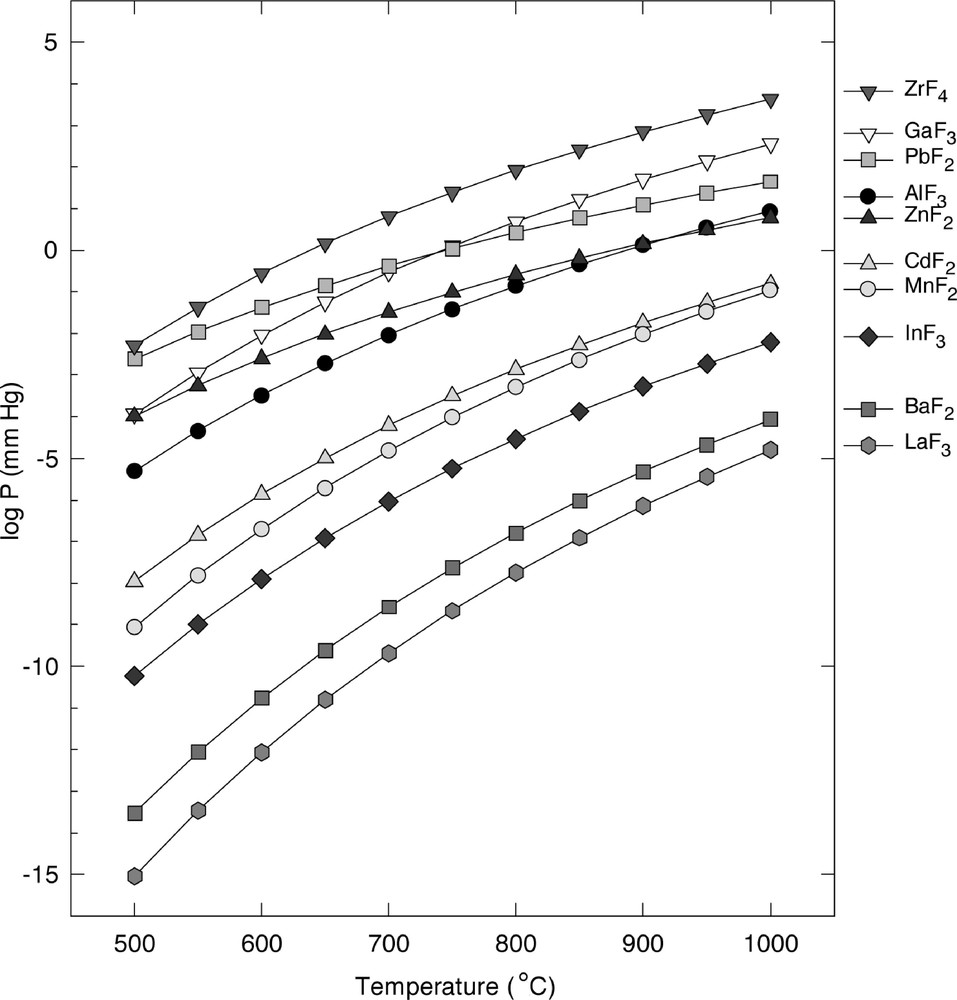
Vapour pressure curves of main metallic fluoride involved in fluoride glasses.
Compositions of starting mixture and evaporated PZG film. The predicted composition is calculated from the vapour pressure curves at 600 °C with Pi = xi Pi (xi is the molar fraction in the melt and P the vapour pressure of the individual fluoride i).
| Composition (mol%) | ||||
| PbF2 | ZnF2 | GaF3 | ||
| PZG starting mixture | 30 | 50 | 20 | |
| Evaporated PZG | experimental | 39 | 19 | 42 |
| predicted | 80 | 8 | 12 |
Owing to the very drastic quenching condition compared to the classical way of preparation of bulk glass from a melt, no stabilising agent such as AlF3, YF3 is in fact necessary to obtain stable vitreous thin films as thick as 50 μm.
The evaporation is conducted under dynamics vacuum (10–4 mbar) and the substrate is heated at 200 °C to insure a good adherence of the film on the susbtrate and to prevent cracks; the deposition rate is about 0.1 μm min–1. In order to minimise the stresses in the deposited film, the thermal expansion of the substrate should be well matched to PZG glass (∼170 × 10–7 K–1); this is the case for CaF2 single crystal and fluoride glass in general.
3.2 Doping with rare-earth ions
As mentioned previously, the main application of the fluoride glass concerns the optical amplification for the telecommunication wavelengths. The only rare earth (RE) candidates for regenerating the optical signal are Pr3+, Tm3+ and Er3+ that have respectively energy levels corresponding to 1.3, 1.47, and 1.55 μm. Owing to the very low volatility of the rare-earth fluorides (similar to LaF3, at least 10 order of magnitude below PbF2), the evaporation of a mixture of PbF2, ZnF2, GaF3 and REF3 is unexpected. In order to solve this problem, we have developed a technique of co-evaporation using one heating source for PZG glass and another one for rare-earth fluoride 〚10〛. The heated substrate is held over the two crucibles, as shown in Fig. 3; the rotation of the sample holder allows a constant doping rate over the whole surface. It is also possible to evaporate a mixture of RE fluoride (Yb–Er, Ce–Er, for example) 〚10〛 to promote energy transfer between ions and thus sensitise certain transitions.
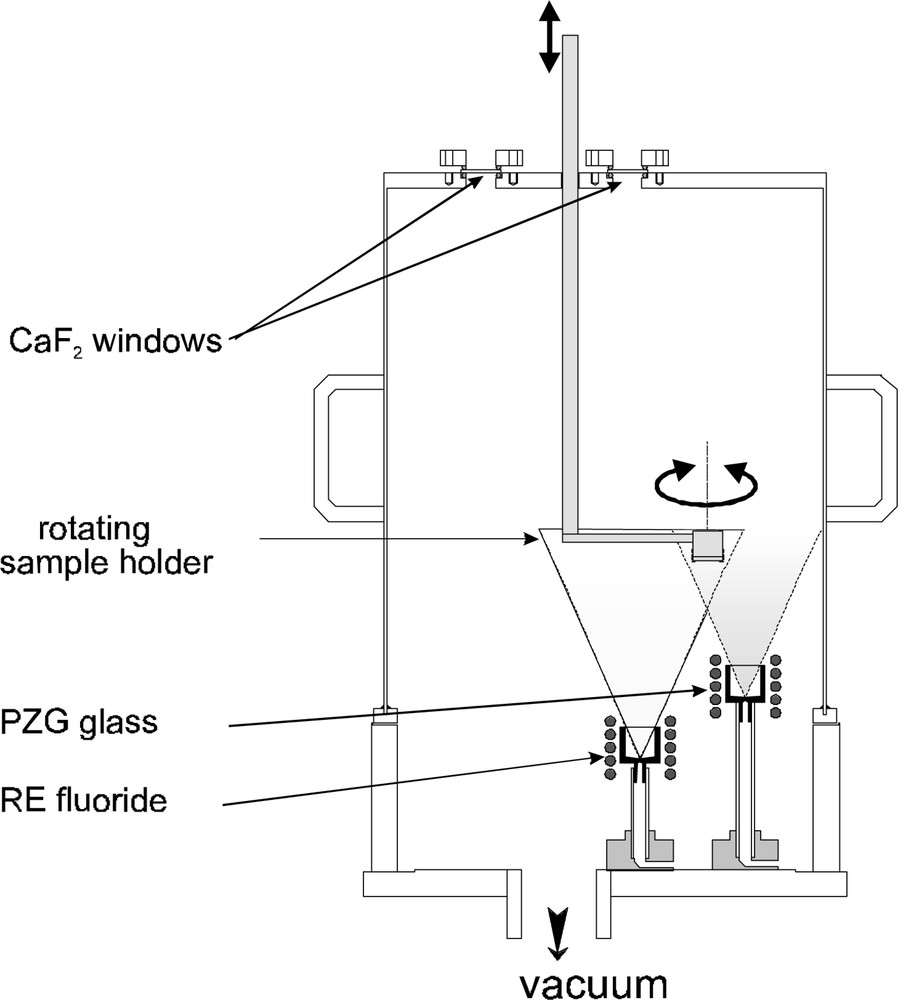
Experimental apparatus for the co-evaporation of PZG glass and rare-earth fluoride.
3.3 Channel waveguide preparation
The miniaturization of the optical devices requires the confinement of the light in channels whose dimensions are reduced to a few microns. A photolithographic process referred as ‘lift-off’ 〚11〛 has been used to create these channels. The various steps are illustrated in Fig. 4. The surface of the substrate – a cleavable CaF2 plate – is coated with a negative photoresist 2- to 4-μm thick. After baking, UV exposure through a mask and development, the ‘over-hang’ resist profiles are obtained. With this geometry, the deposited glass had no chance to stick to the edges of the photoresist, which can be removed easily. In a final step, the substrate is cleaved, leading to injection end-faces with good optical quality. The low propagation loss of the channel waveguides, roughly 0.4 dB cm–1, shows that these structures warrant consideration for use in integrated optics application.
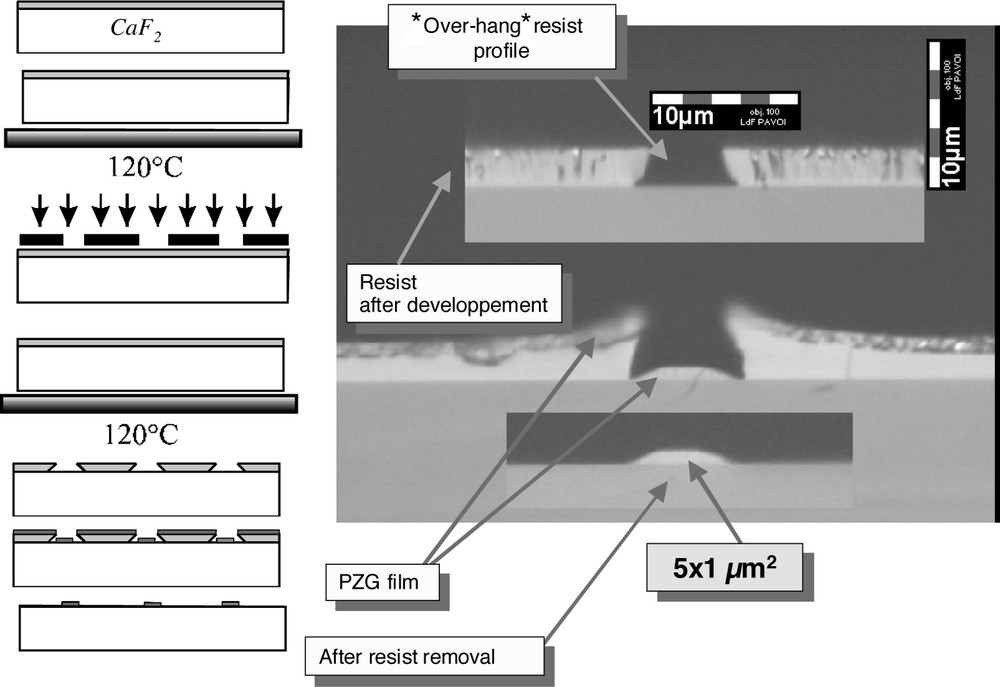
‘Lift-off’ photolithographic process and side view of the PZG channel waveguide (5-μm wide) before and after removal of the photoresist.
4 Active application of optical waveguides
The comparison of luminescence spectra and lifetime of Er3+ for the 4I13/2 → 4I15/2 at 1.55 μm measured on bulk samples and channel waveguides at the same concentration suggests that the environment of the rare earth does not differ from that of samples obtained by quenching from a melt 〚12〛. This result shows that the vapour obtained by co-evaporation is homogenous, free of rare-earth ions aggregates.
Signal gain amplification has been performed around 1530 nm, using a pump beam at 1485 nm (from a laser diode). The signal beam was provided by a tuneable laser diode (1500–1600 nm). A maximum gross gain of 1.6 dB cm–1 has been achieved for 3-mW absorbed pump power at 1530 nm (Fig. 5). However, the propagation losses are still too high to observe a net gain 〚12〛. Improvement will come with the optimisation of the doping rate, the better polishing of the substrate and the higher confinement of light by adding a top layer on the channel waveguide.
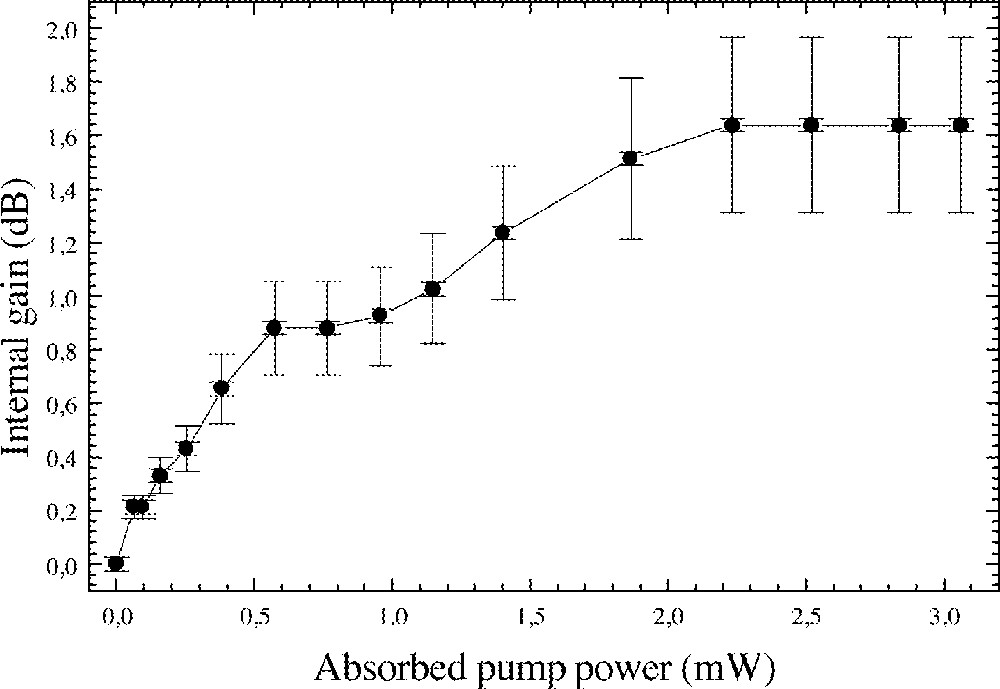
Internal gain at 1.53 μm as a function of the absorbed pump power in Er3+-doped PZG fluoride glass channel waveguide.
5 Conclusion
The PZG vitreous system is quite remarkable owing to its behaviour in the vapour state. This is the only one known that allows the fabrication of stable fluoride glass waveguides by physical vapour deposition. On a structural point of view, the PZG glass obtained by this technique appeared similar to the glass prepared by classical quenching from the melt, as regards to rare-earth luminescence.
By using classical photolithography ‘lift-off’ techniques, channel waveguides have been successfully prepared and optical amplification at 1.55 μm with Er3+ has thus been demonstrated. This new generation of IR devices suggests a route to the development of integrated optics, such as optical amplifiers in local telecommunication systems.


