1 Introduction
Much of the impetus coming from materials chemists to synthesise and study new types of molecular solid has arisen from a desire to create lattice architectures that bring together physical properties not usually associated with simpler continuous lattice materials. Thus some 25 years ago, we showed [1] how it was possible to make organic–inorganic composite salts [2] that were at the same time bulk ferromagnets and optically transparent, with visible colours that changed drastically on passing through the Curie temperature. This concept of associating nouns denoting a property with adjectives that overturn our preconceptions of what is usual can be extended further in the molecular solid state. So, whilst the combination ‘molecular superconductor’ has been valid for 20 years or so, since the discovery of the Bechgaard salts [3], and ‘organic ferromagnet’ since 1991 [4], others such as ‘metallic liquid crystal’ or ‘ferromagnetic molecular superconductor’ still remain to be discovered.
Among the various categories of molecular compounds that have come under scrutiny for their capacity to furnish new properties, charge-transfer salts have been especially prolific. Formed from an aromatic donor cation and an anion that is usually inorganic, they are to be distinguished from charge transfer complexes by the fact that the anion necessarily bears a discrete charge, although the cation:anion stoichiometry often indicates a non-integral mean charge for each cation (e.g., the original Bechgaard salt (TMTSF)2PF6 [3]). Most of the donors have been organo-chalcogen molecules (TTF, BEDT–TTF, etc.), but by varying the inorganic anion it has proved possible to demonstrate practically all the collective electronic ground states known to condensed matter science, such as semiconductors, metals, superconductors, antiferromagnets, spin-Peierls systems, etc., as well as their variation with temperature, pressure and applied magnetic fields. When, in addition, the anion contains a localised 3d (or more exceptionally 4f) magnetic moment, there is a further opportunity for diversity in the properties, as we first showed some 15 years ago [5].
Given that huge worldwide effort over the last 20 years to prepare charge-transfer salts with new properties (for example nearly 100 superconducting phases are now known), it might be thought that the capacity of this class of compounds to generate new structure types and physical properties had been fully explored. However, much novelty is still emerging and the aim of the present article is to outline briefly three examples from the recent work of our group at the Royal Institution. These include the first examples of long-range ordered ferrimagnets, extreme sensitivity of both normal state conductivity and superconductivity to included guest molecules and the first metallic charge-transfer salts that are simultaneously proton conductors.
2 π–d Ferrimagnets
Since Néel set out the theoretical framework of ferrimagnetism within the molecular field approximation over 50 years ago [6], thousands of ferrimagnetic phases have been characterised. In practically all of them, the moments on the two different sites that interact antiferromagnetically but do not cancel are derived from d- or f-electrons. Long-range ordered ferrimagnets based on p-moments are much rarer, though ferromagnetism in decamethylferrocinium – TCNE was observed in 1986 [7]. However, by analysing the crystal-chemical requirements for antiferromagnetic interaction between the two sublattices, my colleague Scott Turner and co-workers have now synthesised a series of charge-transfer salts that do behave as three-dimensionally ordered ferrimagnets, with one sublattice consisting of pπ- and the other of d-electrons [8–12].
At first sight charge-transfer salts of TTF-type donors might not be thought a very promising category of compound in which to search for π–d ferrimagnetism because most such salts contain extended networks of S···S intermolecular contacts. These give rise to significant donor–donor transfer integrals, predominantly in one or two dimensions, so that exchange interactions between pπ-donor and 3d-containing anion are rendered secondary. And indeed it is the case that most TTF and BEDT–TTF salts are quasi-one- or two-dimensional conductors in which the electronic properties of the anion play little part. Examples are the paramagnetic superconductors β"-(BEDT–TTF)4[(H3O)M(C2O4)3]S, with M = CrIII, FeIII [13–18] and S an organic solvent molecule and the ferromagnetic metal (BEDT– TTF)3[MnCr(C2O4)3] [19]. In the latter in particular, the ferromagnetic anion layer proves to have almost exactly the same magnetic parameters as in the insulating phase N(n-C4H9)4[MnCr(C2O4)3] [20]. To induce ferrimagnetism in a lattice AB, on the other hand, we not only wish to construct a three-dimensional lattice, rather the chains or layers, but also to maximise the antiferromagnetic A–B interaction at the expense of A–A and B–B. Fortunately crystal chemistry provides pointers as to how this can be achieved.
In view of the prevalence of S···S intermolecular contacts in organo-sulphur lattices (and even in the chalcogen elements themselves [21]), one obvious strategy is to incorporate S atoms on the periphery of the anion molecule to encourage close contact with S atoms of the donor. That may also have the effect of breaking up the donor stacks or layers. The simplest stable S-containing ligand is NCS–, which fortunately binds to most transition metal ions through N, leaving the S exposed. In numerous salts such as (BEDT–TTF)4[Fe(NCS)6]·CH2Cl2 [22] and (BEDT–TTF)2[Cr(NCS)4(NH3)2] [23], the NCS group do indeed penetrate into the donor-cation layers, creating donor packing motifs not found before, and short donor-anion S···S distances are found. Nevertheless, these salts are almost perfect paramagnets with Weiss constants below 1 K. However, on modifying the isothiocyanato-anion to incorporate a mono- or bi-dentate aromatic N-donor ligand such as iso-quinoline or 1,10-phenanthroline, a series of salts emerges, in which donor–donor contacts have indeed been minimised, and donor-anion ones enhanced. An example of the crystal structure [24] and magnetic behaviour [8] of one of these is illustrated in Figs. 1 and 2. It remains to be seen whether the same strategy can be extended to other types of complex anion with conjugated ligands.
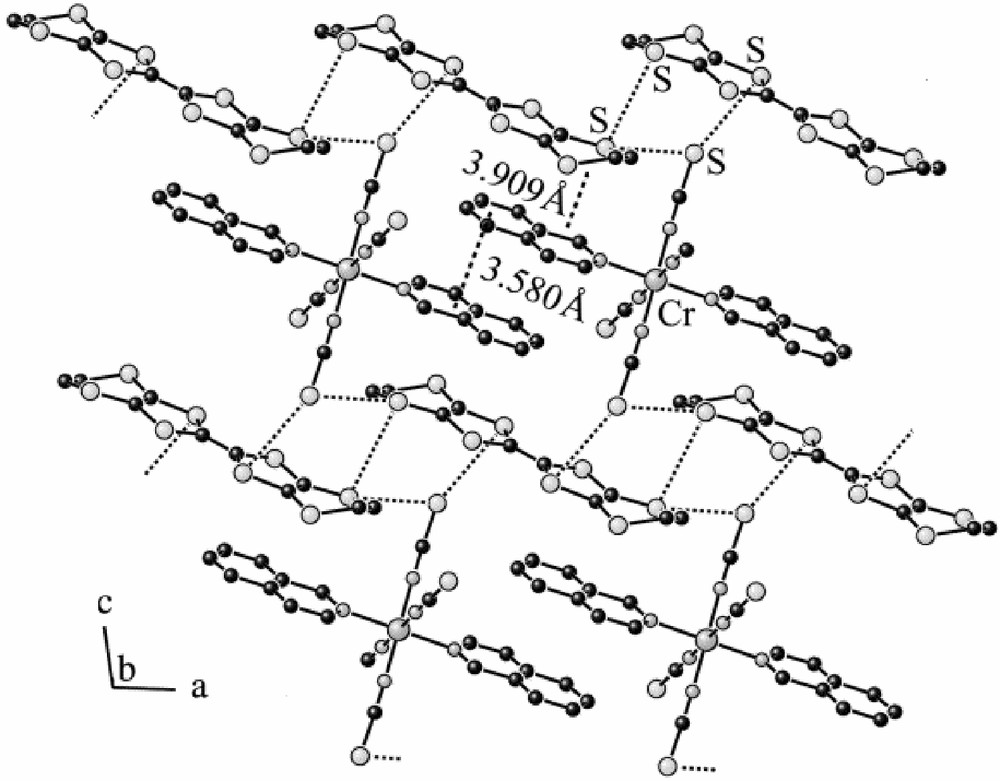
The crystal structure of (BEDT–TTF) [Cr(isoquinoline)2(NCS)4] [24].
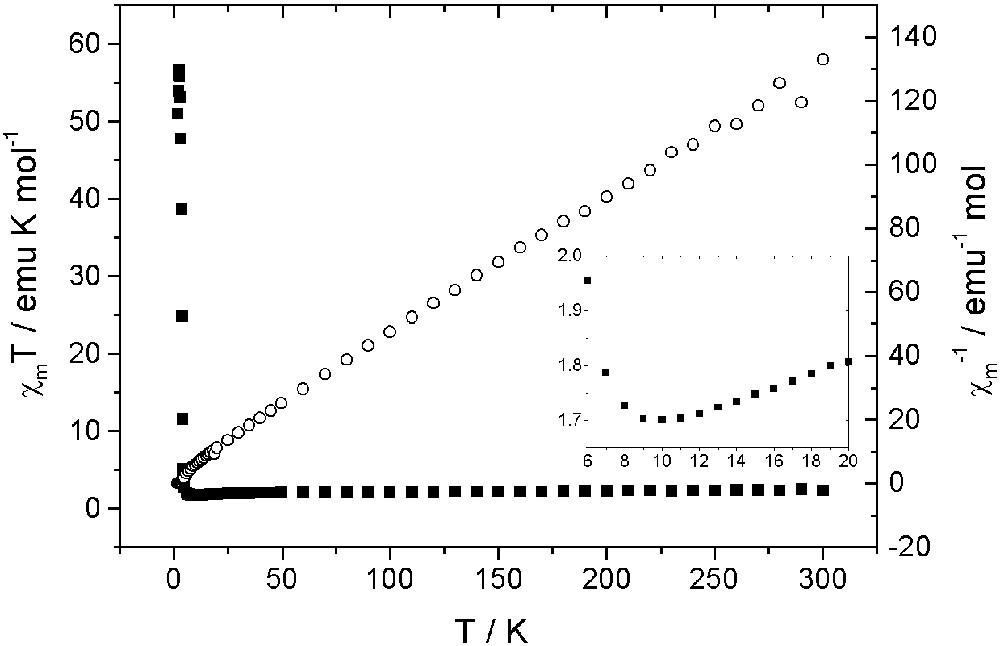
Temperature dependence of magnetic susceptibility of (BEDT–TTF)[Cr(iso-quinoline)2(NCS)4] [8]. Note the minimum, corresponding to antiferromagnetic near-neighbour exchange.
3 Guest molecules in molecular superconductors
Superconducting charge-transfer salts sometimes contain guest molecules in interstices within the crystal lattice, incorporated from the solvent used in the electrocrystallisation procedure. For example, in 1988 we reported the first molecular superconductor (and in fact the first superconductor or any kind) containing water, (BEDT–TTF)3Cl2·2 H2O [25]. With complex anions, cavities may appear in the lattice that are capable of accommodating larger molecules but a priori one would not expect such adducts to have any direct effect on the bulk electronic properties. However, a recent example reveals how extremely subtle structural changes accompanying a change of guest molecule can have a substantial effect on the collective ground state. This finding is particularly surprising because the orbitals of the guest molecule do not contribute in any way to those forming the Fermi surface.
Since we first reported them in 1995 [13, 14], we have extended the series of superconductors β"-(BEDT–TTF)4[(H3O)M(C2O4)3]·S both as regards the metals M (Cr, Fe, Ga) and guest molecules S(C6H5CN, C6H5NO2, C5H5N, C6H5CH2CN, CH2Cl2) [15– 18, 26–28]. The structures of all the compounds consist of alternate layers of BEDT– TTF with a mean charge of +0.5 per molecule and layers containing [(H3O)M(C2O4)3]·S. The anionic layer is a pseudo-hexagonal array of [M(C2O4)3]3– and H3O+ with the guest molecule S occupying cavities formed by the O-atoms of the oxalato-ligands. Where S contains an aromatic ring, its plane makes an angle between 30°–40° to the plane defined by the M. Two examples of anion layers containing different guest molecules are shown in Fig. 3a (M = GaIII; S = C6H5NO2, C5H5N). Fig. 3b illustrates the alteration of donor and anion layers in the same salts, emphasising that the two structures are almost identical: they share the same space group (C2/c) and their unit cell volumes differ by only 1.2% (290 K) [27]. In contrast, the electron transport properties of the two salts diverge dramatically, both in respect of the normal state and superconductivity state.
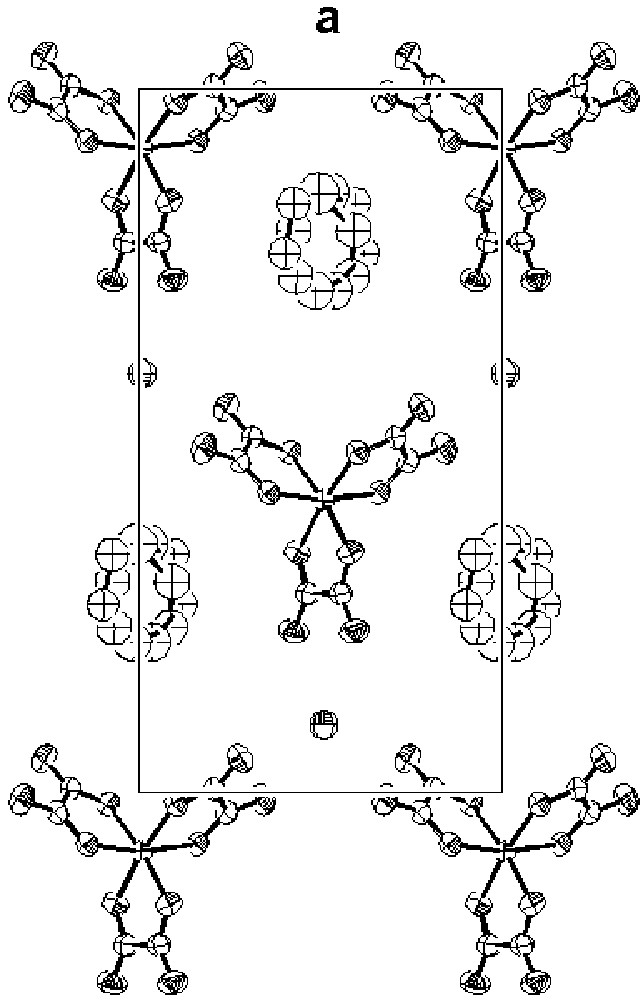
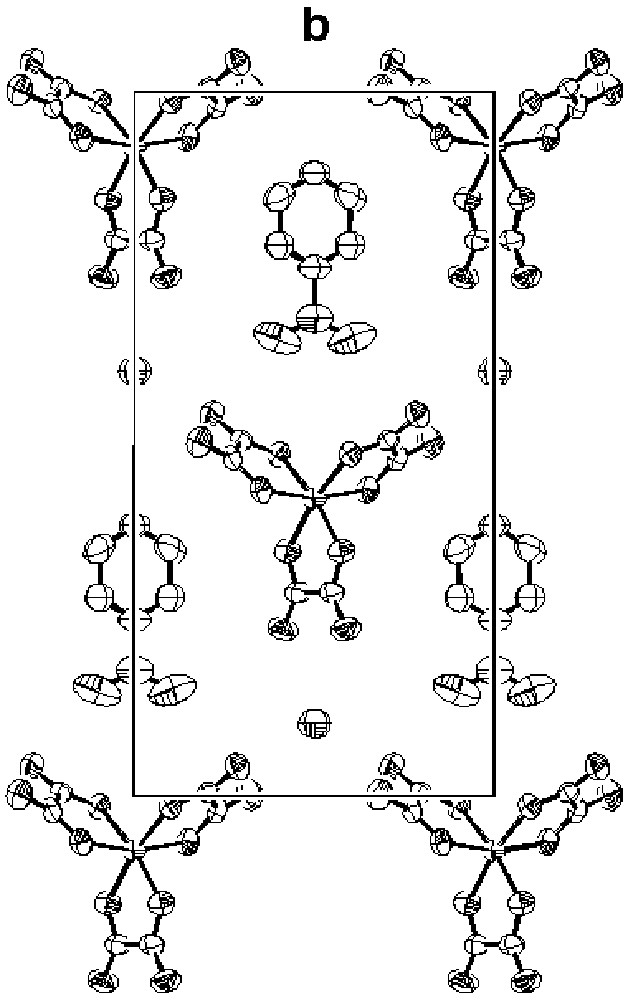
The anion layers in β"-(BEDT–TTF)4[(H3O)Ga(C2O4)3].S [27]; (a) S = pyridine; (b) S = nitrobenzene.
First of all, the resistance of the M = Fe; S = C6H5CN salt decreases monotonically with decreasing temperature from 300 to 8.3K, where it becomes superconducting [14]. In contrast, in both the M = Fe, Ga; S = C5H5N and M = Ga; S = C6H5NO2 salts, the resistance reaches a minimum of about 130 and 150 K respectively, and then rises again at lower temperatures [16, 27]. The resistance of the M = Ga; S = C5H5N salt reaches a maximum at about 60 K, decreasingagain down to a probable superconducting transition below 2 K, whilst the resistance of the M = Ga; S = C6H5NO2 salt continues to rise till it reaches about 9 times its 300-K value at 7.3 K, whereupon there is an abrupt transition to the superconductingstate. The contrasting behaviour of the M = Ga salts with S = C5H5N and C6H5NO2 is shown in Fig. 4 [27], where the resistances are normalised at 300 K.
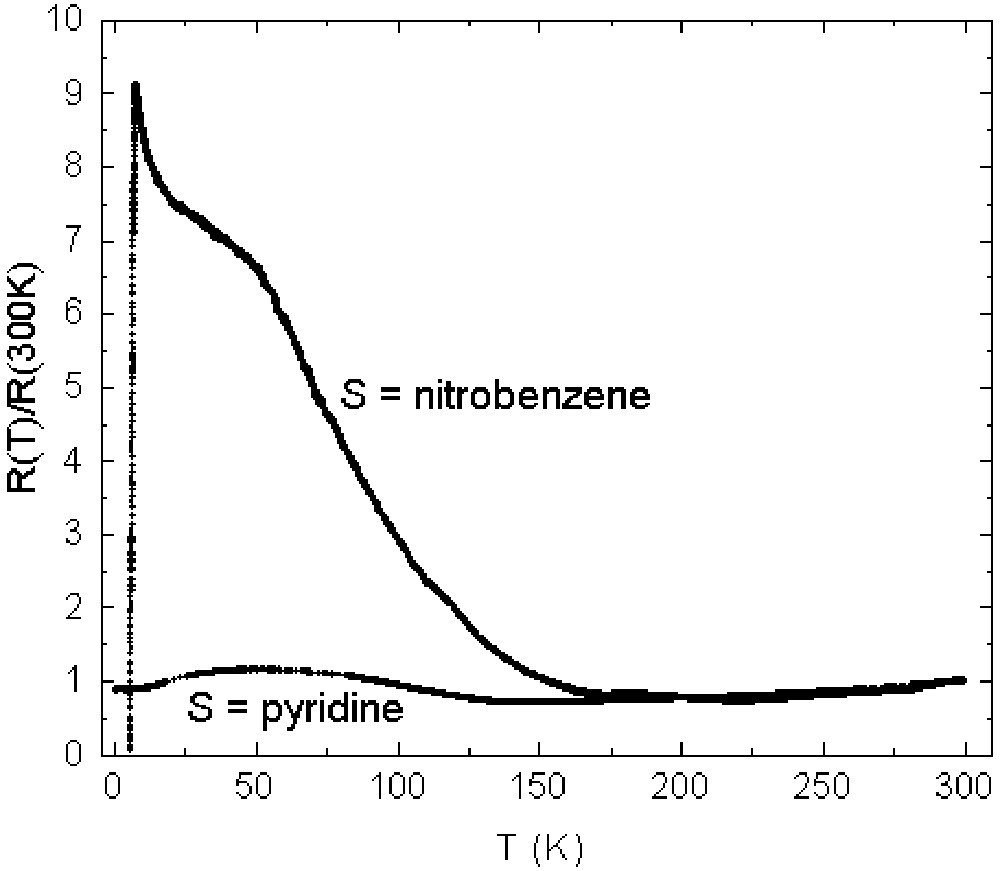
Temperature dependence of resistance of β"-(BEDT–TTF)4[(H3O)Ga(C2O4)3]·S parallel to the conducting planes (S = pyridine, nitrobenzene) [27].
Searching for the origin of this dramatic contrast, two subtle differences in the structures may be relevant. First, at 290 K the pyridine molecule is orientationally disordered (Fig. 3a), while the nitrobenzene molecule is not. Second, there is a small difference between the conformation of the terminal –CH2CH2– on one of four crystallographically inequivalent BEDT–TTF in each unit cell. At 290 K, there is considerable positional disorder, corresponding to inversion of the twisted conformation in both salts, but whereas the disorder is frozen out at 100 K in the nitrobenzene salt, it remains fully present at the same temperature in the pyridine case [27]. Most probably this is because there is more space in the latter for the –CH2CH2– group to liberate because there is no side-chain attached to the aromatic ring.
Preliminary measurements of Schubnikov–de Haas magnetoresistance oscillations indicate a well-developed Fermi surface at low temperature in both salts with quite similar area [29, 30]. Furthermore, it is known from temperature-dependent single crystal X-ray diffraction measurements that the angle between the plane of the phenyl ring and the anion plane in the nitrobenzene salt varies from 32° at 290 K to 40.5° at 40 K as the lattice contracts and the cavity that it occupies diminishes [31]. Clearly, extremely subtle factors are at work here to determine the contrasting behaviour of these two closely similar salts.
4 Charge-transfer salts containing proton channels
Electron transport is the factor that has driven the effort towards synthesising new charge-transfer salts over the last two decades, but recently there has been increasing emphasis on what have been called multi-functional materials, i.e. ones combining two desired properties, such as magnetism and conductivity, if possible in synergy with one another. Amongst the many different properties sought, ionic conductivity has hardly ever been mentioned, despite the fact that combined electronic and ionic conductivity is a crucial requirement for materials used as electrodes in solid-state batteries. Recently, however, we have found that structures of the type described in the previous section are capable of being modified to introduce channels of crown ether molecules, inside which are found both H3O+ and H2O, such that proton migration can easily take place [32, 33]. As we shall describe now, such salts are indeed both protonic and electronic conductors.
In common with many members of the BEDT–TTF charge transfer salt family, the series β"-(BEDT–TTF)4[(H3O)M(C2O4)3]·S contain alternating layers of donor cations and inorganic anions. The stacking sequence of the layers can be symbolised as ABAB’ABAB’..., where the prime indicates reversal in the stereo-configuration of the tris-oxalato-metallate ions, which are all ▵ in one layer and all Λ in the next [17]. In the new series of compounds, though, alternate layers of BEDT–TTF are replaced by 18-crown-6, so that the stacking sequence becomes ABCB’ABCB’... Overall, the stoichiometry is (BEDT–TTF)4[M(C2O4)3]2(18-crown-6)(HxO)9, x being 2 or 3, and M = Fe, Cr or Ga [32, 33] (Fig. 5). The packing motif of the BEDT–TTF layers (A) remains β", as in the superconductors in section 3 above, and analysis of the bond lengths using the well-known relation with charge [34], together with the C=C Raman frequencies, indicates a mean donor charge of +0.5, just as in the superconducting salts. One water molecule is also present in the cavity formed by the oxalate O and, also by analogy with the superconducting salts, can be presumed to be H3O+. Overall charge balance in the lattice, therefore, requires that of the remaining eight water molecules situated in the crown ether layer C, two must be H3O+ and five H2O. The crown ether molecules in layer C are stacked with their mean planes parallel, forming an array of parallel channels within which reside the water molecules (Fig. 6). Some of them lie close to the centre of the channel, while others are found near the walls. It is not possible to identify from the crystallographic refinement which water molecules are H3O+ and which are H2O, but it appears probable that the ones closest to the crown ether O atoms are the H3O+. The O···O distances between the water molecules inside the channels (1.84, 1.97 and 1.47Å) strongly suggest that they are H-bonded, with the abnormally short distance being due to the disorder of one O over two sites.
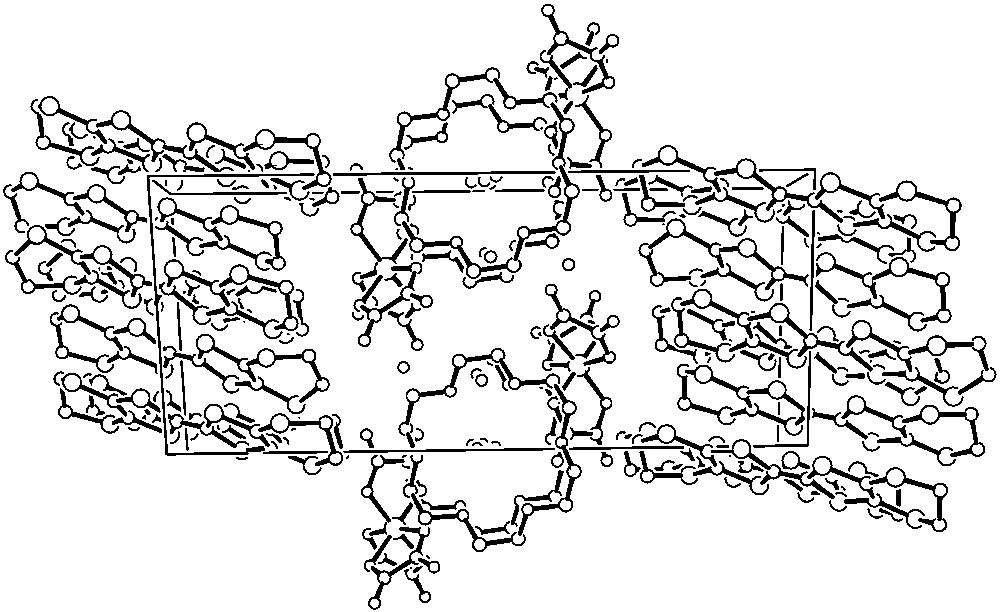
The crystal structure of β"-(BEDT–TTF)4[Ga(C2O4)3]2(18-crown-6)(HxO)9, showing the sequence of cation, anion and crown ether layers [33].
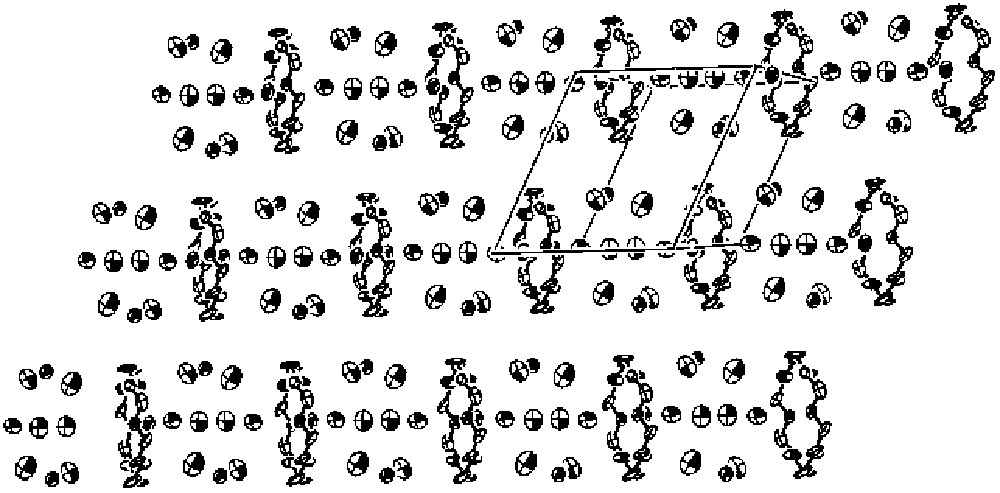
The crown ether and water layers in β"-(BEDT–TTF)4[Ga(C2O4)3]2(18-crown-6)(HxO)9.
Like all the other β"-BEDT–TTF salts with mean donor charges of +0.5, this new series of compounds are metallic at room temperature with conductivities of 300, 200 and 500 S cm–1 for M = Cr, Fe, Ga, respectively. In all cases, however, the resistance reaches a minimum (at 190, 240 and 110 K for M = Cr, Fe, Ga respectively) followed up a gradual upturn at lower temperature [33]. The magnetic susceptibilities of the Cr and Fe salts are dominated by the metal ions, being Curie–Weiss paramagnets with vanishingly small Weiss constants, while the Ga salt exhibits a temperature-independent Pauli paramagnetism amounting to 4.0 × 10–4 emu mol–1, as anticipated for a metal. To determine ionic conductivity, it is necessary to use electronically blocking but ionically conducting electrodes, and, in preliminary experiments on compressed pellets with Nafion electrodes, substantial conductivity was observed, increasing with increasing temperature from 230 to 310 K [35]. The measurements were made with an impedance analyser (HP 4194A) at frequencies from 100 Hz–5 MHz. For comparison, a pellet of a BEDT–TTF salt that is not expected to be ionically conducting, (BEDT–TTF)2(ClO4), was recorded under the same conditions. It proved to have a resistance at 270 K about 103 higher than the crown ether salts. Further experiments are in progress, but it appears that this new family of BEDT–TTF compounds are the first molecular charge-transfer salts to be protonic as well as electronic conductors.
5 Conclusion
The principal conclusion of this brief survey of ongoing work on BEDT–TTF charge-transfer salts must be that this class of molecular solids, which is already very extensive after two decades of study, remains capable of generating surprising novelties. When molecules are assembled in new solid-state architectures, new and often unexpected properties emerge. In the present case, we have the first π–d molecular ferrimagnets, superconductors whose properties are dramatically modified by changing small guest molecules in the lattice and molecular metals that also transport protons. There can be no doubt that future synthetic ingenuity will reveal others, to the enrichment of chemistry, physics and materials science.
Acknowledgements
The work described here has been carried out by many talented and enthusiastic co-workers, whose names appear in the references. To them I express my warmest appreciation. The Royal Institution group has been supported for many years by the UK Engineering and Physical Sciences Research Council. Financial support from the European Commission (TMR Network on Molecular Magnetism; COST Action D14) and the Japan Society for the Promotion of Science is gratefully acknowledged.


