1 Introduction
In the past decade, gene therapy definitely became one of the fastest-developing fields of biomedical research. It offers a conceptually novel therapeutic strategy for the treatment and cure of acquired diseases like cancer [1] and inherited diseases such as cystic fibrosis [2].
Although a wide array of physical, chemical and biological methods are available for transferring genes into cells, none of the gene-delivery vectors known to date can be referred to as an ideal vector. Nevertheless, some of them show promising possibilities.
An ideal vector should be highly efficient in delivering the gene in a target-specific manner, stable in vitro as well as in vivo, non-toxic, non-immunogenic, easy prepared in large quantities and it should protect the gene from nuclease degradation.
The cationic liposomes are non-viral most widely used vectors [3], owing to their capacity in packaging well the DNA fragments whit their positive charge to form cationic liposomes/DNA complexes. They have many important qualities such as being much less or non-immunogenic and non toxic, have no know limitation in the size of DNA, can be custom-synthesized for targeting and easy scalable for large scale production.
The DC-Chol 1, (3β-[N-(N′,N′-dimethylamino-ethane)carbamoyl]-cholesterol, see Fig. 1), is one of cationic lipid more used in the preparation of liposomes in combination with DOPE, 2, (dioleoyl-phosphatidyl-ethanolamine, see Fig. 1), for the delivery of nucleic acids fragments in the gene therapy [4, 5]. US FDA and the regulatory authorities of other countries for use in clinical trials approved it.
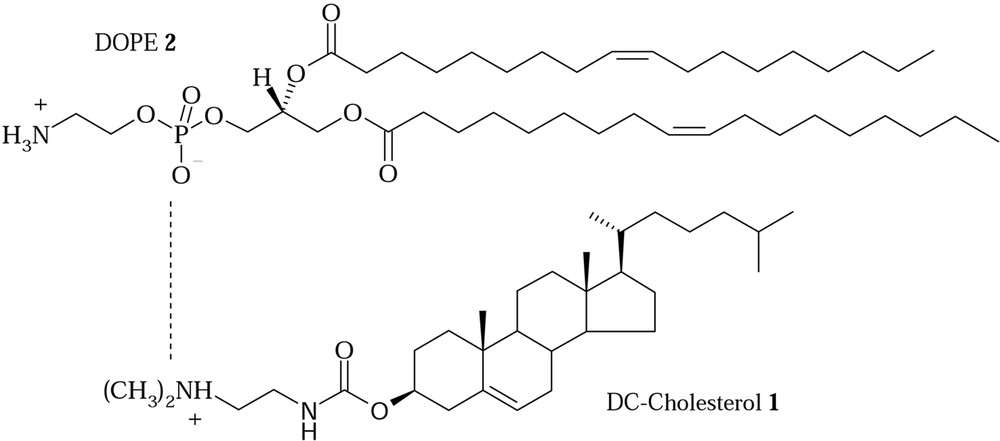
There are useful features that make DC-Chol high in the list of cationic lipids for gene delivery. It forms a stable liposomal formulation with DOPE that can be stored for months at 4 °C without any change in size or lipid degradation [6]. DC-Chol/DOPE liposomes show a better transfection activity than other cationic lipids formulations in vivo [7,8].
2 Results
The synthesis of DC-Chol now described in the literature is based on the treatment of cholesterol with phosgene derivatives [9]. The obtained cholesteryl chloroformiate is then treated with N,N-dimethyl-ethylene-diamine, in order to get the final product, with a reported overall yield of 21% [10].
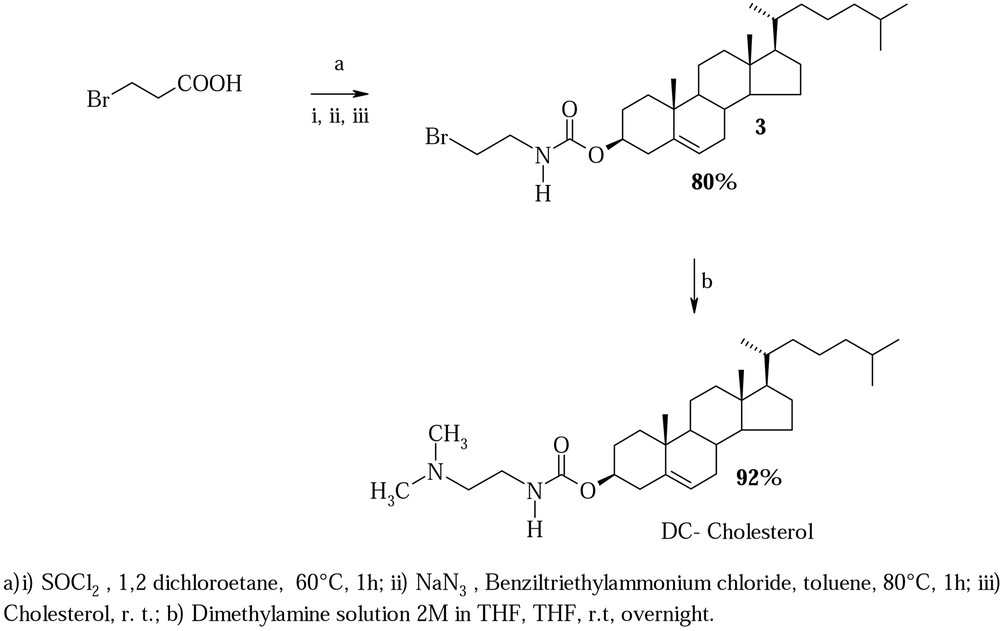
We propose a great improvement in the synthesis of DC-Chol, using a modified Curtius reaction, according to the methodology used for the synthesis of methyl urethanes [11,12].
The procedure, reported in Fig. 2, is as follows: 3-bromo-propionic acid (1 mmol) was treated with SOCl2 in dry 1,2 dichloroethane at 60 °C for 1 h, until its complete transformation into the corresponding acid chloride. Volatile material were eliminated under educed pressure, dry toluene (5 ml) and dry benzyl-triethyl-amonium chloride (0,1 mmol) were added with stirring. Dry sodium azide (2.4 mmol) was added in portions (4 × 0.6 mmol) over 1 h keeping the reaction temperature at 60 °C under stirring. The solution was kept for 15 min at 80 °C. Cholesterol (5 mmol) was then added and the reaction mixture allowed to stand over night at room temperature. The solution was diluted with ethyl ether (50 ml) washed with water and dried with anhydrous sodium sulphate. Solvent removal gave intermediate 3 that can be used in the successive step without purification. For analytical purposes, crude 3 was purified on silica gel, eluting with CHCl3/MeOH 98:2 (80% yields measured on purified compound) and characterized by 1H- and 13C-NMR.
1H-NMR, CDCl3 Varian Gemini 200: δ : 0.69 (s, 1H, 18-Me), 0.86 (d, 6H, 26-Me-27-Me), 0.91 (d, 3H, 21-Me), 1.03 (s, 3H, 19-Me), 3.48 (bq, 2H, CH2NH), 3.58 (m, 2H, CH2Br), 4.53 (m, 1H, H-3), 5.05 (m, 1H, NH), 5.41 (d, 1H, H-6). 13C-NMR, CDCl3, δ: 11.93, 14.18, 22.75, 22.89, 26.80, 27.30, 28.09, 28.27, 29.26, 29.40, 29.75, 29.84, 31.98, 35.88, 36.27, 36.65, 38.68, 39.59, 39.82, 42.40, 45.57, 45.86, 50.11, 53.21, 53.93, 36.22, 56.77, 129.79, 130.12, 156.22. Requires: C 67.15%, H 9.39% Br 14.89% N 2.61%. Found: C 67.01% H 9.52% Br 14.67% N 2.55%.
Crude 3 was dissolved in 3 ml of THF. 1 ml of 2M solution of dimethylamine in THF was added and the reaction mixture left at room temperature overnight. After elimination of volatile materials under reduced pressure, crude 1 was purified by chromatography on silica gel in CHCl3/MeOH 9:1, affording pure DC-Chol 1 with 92% yield that resulted identical to an authentic sample. The total yield of the described protocol with any intermediate purificated is about 74%.
The synthetic strategy, reported in Fig. 2, is characterized by a good flexibility, suggesting the possibility to synthesize a wide variety of DC-Chol derivatives, by modifying the used halogen-acid and the amine. In addition, the described protocol avoids the use of phosgene and its derivatives, used for the preparation of cholesteryl chloroformiate.


