1 Introduction
Magnetic clusters are a class of functional molecules of current interest in molecular magnetism and in other related areas such as molecular electronics. The main reason for this interest is due to the capability of these systems to act as single molecule magnets exhibiting at low temperature magnetic bistability [1]. This feature opens the possibility to use these nanomagnets in molecular electronics as a means to create high-memory magnetic components. In order to reach this goal, organized assemblies of these molecules into one-, two- and three-dimensional arrangements need to be obtained. Here we show two possible ways to organize magnetic molecular clusters into two dimensions. In the first part, we discuss the use of the Langmuir–Blodgett (LB) technique in order to obtain organized magnetic films formed by monolayers of these clusters. Although the LB technique is a very attractive molecular assembling method, this technique suffers from certain inherent limitations that prevent its generalized use or its extension to large-scale applications. Development of other methods is therefore necessary. In this context, an alternative method is that of preparing self-assembled monolayers (SAMs). Some preliminary results obtained using this method are reported in the second part of the work.
2 Langmuir–Blodgett films
The Langmuir–Blodgett technique has recently been used to organize polyoxometalate clusters [2]. Extension of this technique to Mn12 appears very desirable to explore the fabrication of organized films of the aforementioned single-molecule magnets. Two strategies have been used with this aim. The first one consists of mixing Mn12 acetate (Mn12Ac) or benzoate (Mn12Bz) derivatives with a lipid molecule, while the second procedure is based on the use of Mn12 derivatives specifically designed to form LB films.
Using the first procedure, we have shown that an homogeneous Langmuir monolayer is obtained from mixtures of Mn12Ac/BA or Mn12Bz/BA, where BA = behenic acid (CH3(CH2)20COOH) [3]. On the contrary, when behenic alcohol is used as matrix, the Langmuir monolayer is not formed. Instead, crystallites of Mn12 are observed at the water–gas interface. One possible explanation of the different behaviour of behenic alcohol and behenic acid is the effect of the polar heads of these amphiphilic molecules to stabilize the Mn12 cluster. Whereas the carboxylic function of behenic acid can interact strongly or even substitute the acetate or benzoate groups of the Mn12 clusters, the alcohol groups interact weakly. Probably the partial substitution of the acetate and benzoate groups by behenate stabilizes the Mn12 clusters at the water–gas interface and allows the formation of a well-defined monolayer.
The Langmuir films so formed are perfectly stable versus time if the ratio lipid:cluster is high enough (typically higher or equal to 5). Transfer of the monolayer onto a solid hydrophobic substrate is easily achieved at a surface pressure of 30 mN m–1. A Y-type LB film is then obtained with a transfer ratio close to 0.90–0.95. The structures of these films, investigated through X-ray diffraction and IR measurements, show that lamellar structures with the clusters organized in well-defined monolayers are obtained (Fig. 1). As expected, these LB films show magnetic hysteresis at low temperatures with coercive fields of ca. 0.1 T (benzoate derivative) or 0.06 T (acetate derivative), which vanish as the temperature is increased to 5 K. We also observe that in the benzoate derivative the shape of the loop depends on the orientation of the film with respect to the applied magnetic field H. Thus, when H is parallel to the plane of the magnetic monolayer, the loop is softer than when H is perpendicular (Fig. 2). Such an anisotropy indicates a preferential orientation of these anisotropic clusters within the layers. This effect is not observed for the acetate film that seems to be less oriented within the layer.
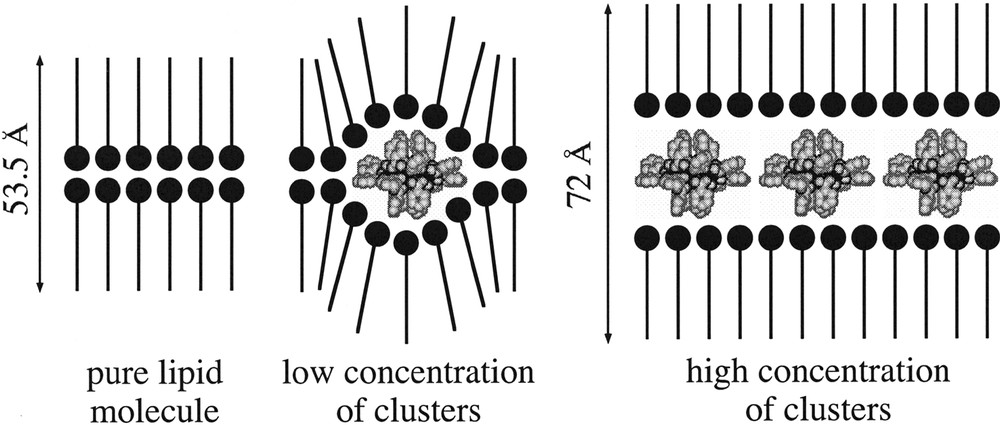
Schemes of the Y-type Langmuir–Blodgett films formed when the lipid ratio is varied.
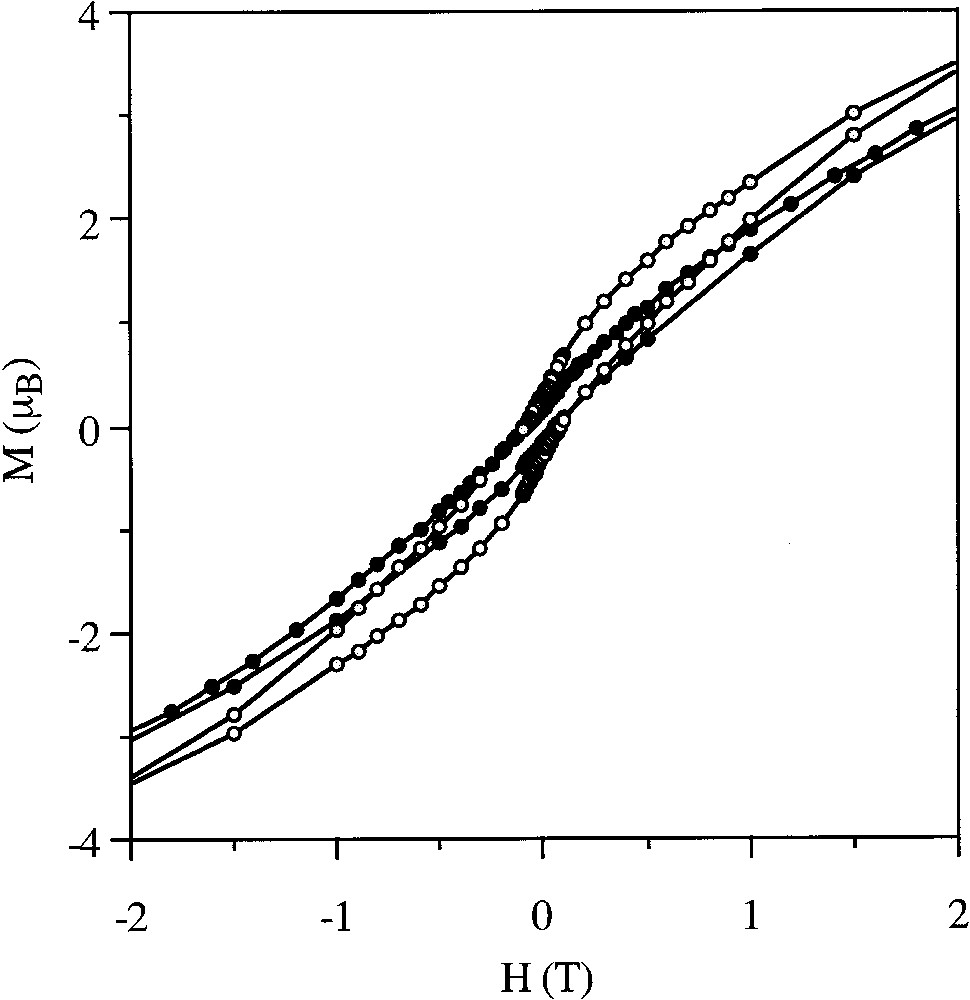
Hysteresis loops at 2 K of the Langmuir–Blodgett film prepared with the Mn12Bz cluster and behenic acid with a cluster/lipid ratio of 1:10. Magnetic field perpendicular (filled circles) or parallel (empty circles) to the film layer.
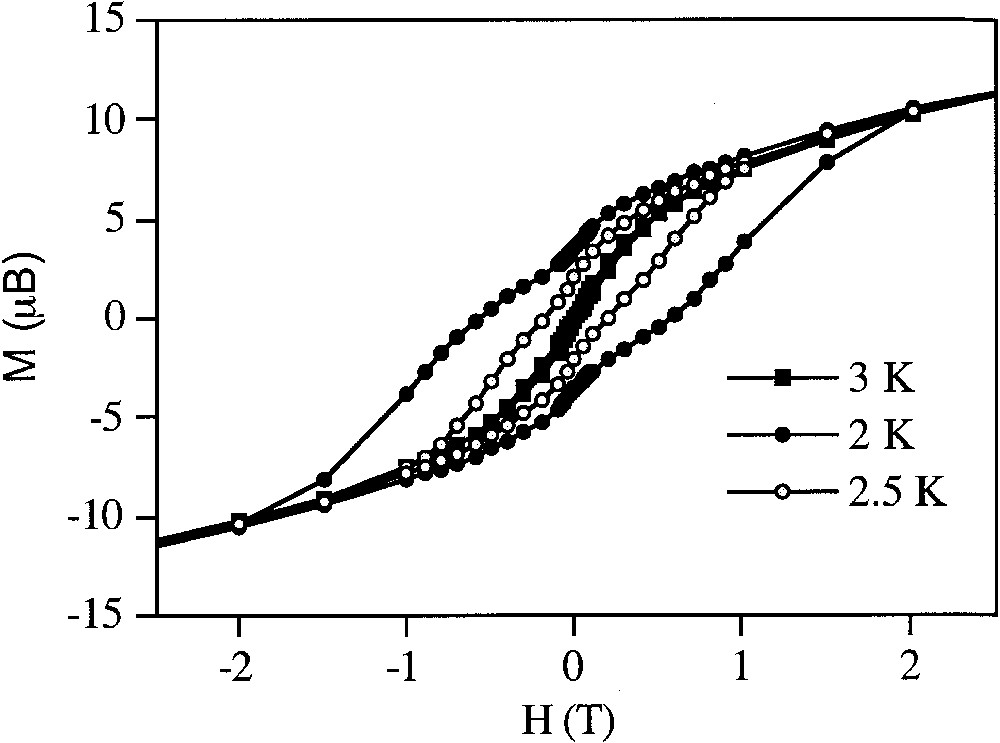
The second procedure is based on the use of stearate Mn12 derivatives. The synthesis of alkyl chain derivatives of inorganic complexes is one of the most common strategies used to form Langmuir films. We have used such a procedure to avoid the use of an organic matrix. The ligand we have chosen is the stearate ligand (CH3(CH2)16COO–) because it presents a carboxylic group to coordinate the Mn12 cluster and an organic chain long enough to form a Langmuir film at the water–gas interface. We have synthesized a derivative of formula [Mn12O12(O2C(CH2)16CH3)16(H2O)4] as deduced from chemical analysis. Magnetic measurements of this brown powder show a magnetic behaviour characteristic of Mn12 clusters. It presents a hysteresis loop of magnetization with a coercive field of 1.15 T at 2 K (Fig. 3) and out-of-phase peaks in the AC susceptibility data between 2 and 10 K that shift towards higher temperatures at higher frequencies (Fig. 4). When a CHCl3 solution of this cluster is spread on pure water, the compression isotherm shows values of the area per molecule much smaller than the values we should expect for this cluster. This behaviour suggests the formation of multilayers rather than a monolayer. Brewster angle microscopy images confirm this hypothesis. Still, the reflectivity observed is too high for a monolayer. It should correspond to multilayers of the cluster that self-organize at the water–gas interface. In a second step, these multilayers have been transferred onto a solid substrate. X-ray measurements show that the periodicity of these films corresponds to the periodicity of stearic acid. This indicates that the cluster decomposes partially, liberating stearate molecules after spreading at the water–gas interface. These stearate molecules constitute the periodic part observed by X-ray diffraction. The Mn12 cluster is present within the film, as it is shown by the intense brown colour of the film, but it is not well organized. Magnetic measurements of these films show a marked hysteresis loop of magnetization with a coercive field of 6000 G at 2 K (Fig. 5).

Hysteresis loops of magnetization of the compound [Mn12O12(O2C(CH2)16CH3)16(H2O)4] at different temperatures.

AC susceptibility vs. T plot of the compound [Mn12O12(O2C(CH2)16CH3)16(H2O)4] at different frequencies. Full and empty symbols correspond, respectively, to the in-phase and out-of-phase components of the AC magnetic susceptibility.
Hysteresis loops of magnetization of the LB film of the compound [Mn12O12(O2C(CH2)16CH3)16(H2O)4] at different temperatures.
In order to stabilize the stearate Mn12 cluster at the water–gas surface, this cluster has been mixed with stearic acid in 1:5 and 1:10 cluster/lipid ratio and spread on pure water. The areas per molecule are much bigger than for the pure cluster and indicate the formation of a monolayer. Then, LB films of this mixture have been transferred onto a solid substrate. These films are magnetic and show a hysteresis loop with a coercive field of 1600 G at 2 K.
The behaviour of this cluster may serve to better understand the behaviour of the mixtures of the acetate and benzoate derivatives with behenic acid at the water–gas interface. From IR measurements, we have seen that there is partial substitution of the acetate and benzoate groups by the behenate molecules co-spread on the water-gas interface. This substitution cannot be complete, because in that case we should observe the formation of multilayers, as in the stearate derivative. Thus, it seems that the stabilization of the Mn12 clusters on the water–gas interface is due to a partial substitution of acetate or benzoate ligands by the lipid carboxylate.
3 Self-assembled monolayers
In 1946 Zisman prepared a monolayer by adsorption of an alkylamine molecule on a solid support of platinum [4]. The driving force of this spontaneous process is the strong interaction of the amino group of the adsorbate with the substrate (the metallic surface). This technique has been extensively used to create highly ordered self-assembled monolayers. There are several types of SAMs, e.g. alkyltrichlorosilanes on glass [5], fatty acids on metal oxide surfaces [6] and sulphur-containing adsorbates (thiols, disulfides and sulphides) on gold [7]. The latter class of monolayers has widely been studied during the past 20 years. In general, they are stable due to the strong sulphur-gold bond, so they have found some useful applications [8]. Furthermore one can form these SAMs either by direct attachment of a thiolated molecule on the gold surface [9], or by secondary self-assembly of a molecular monolayer onto a thiolated SAM, which is directly attached to the gold surface (Fig. 6) [10]. In this last case, the two monolayers have opposite charges, so the driving force of this process is purely electrostatic.

Schematic illustration of the deposition of multilayers on a gold surface by electrostatic interactions.
In order to build SAMs from Mn12 magnetic clusters, both approaches have been used. In a first step, we tried to functionalise Mn12 with a carboxylic acid containing thiol groups. Thioctic acid (1,2-dithiolane-3-pentanoic acid, 1, see Fig. 7) was used as the cyclic disulfide functionality of this acid affords two anchoring points to the gold surface per chemisorbed molecule, a fact that has shown to produce more stable SAMs [11]. However, the magnetic cluster seemed to be unstable in the presence of this acid and decomposed when organic solutions containing Mn12O12(CH3COO)16(H2O)4 and thioctic acid as reactants were stored for a few days for crystallisation. Still if this mixture is immediately evaporated to dryness, a magnetic film, which is insoluble in all common solvents, was obtained (Fig. 8). In view of the magnetic properties, this film contains Mn12 clusters. Thus, an out-of-phase frequency-dependence magnetic susceptibility signal was observed in the 3–9-K range (Fig. 9). Interestingly, a magnetic hysteresis with a large coercive field of ca. 1 T was observed at 2 K (Fig. 10). We also tested other carboxylic acids that have shown some ability to be bound to gold surfaces such as 3-mercaptopropionic acid, 2 and isonicotinic acid, 3 (Fig. 7). These attempts were unsuccessful.

1,2-dithiolane-3-pentanoic acid, (thioctic acid) (1), 3-mercaptopropionic acid (2), Isonicotinic acid (3), sodium 2-mercaptoethanesulfonate (4).
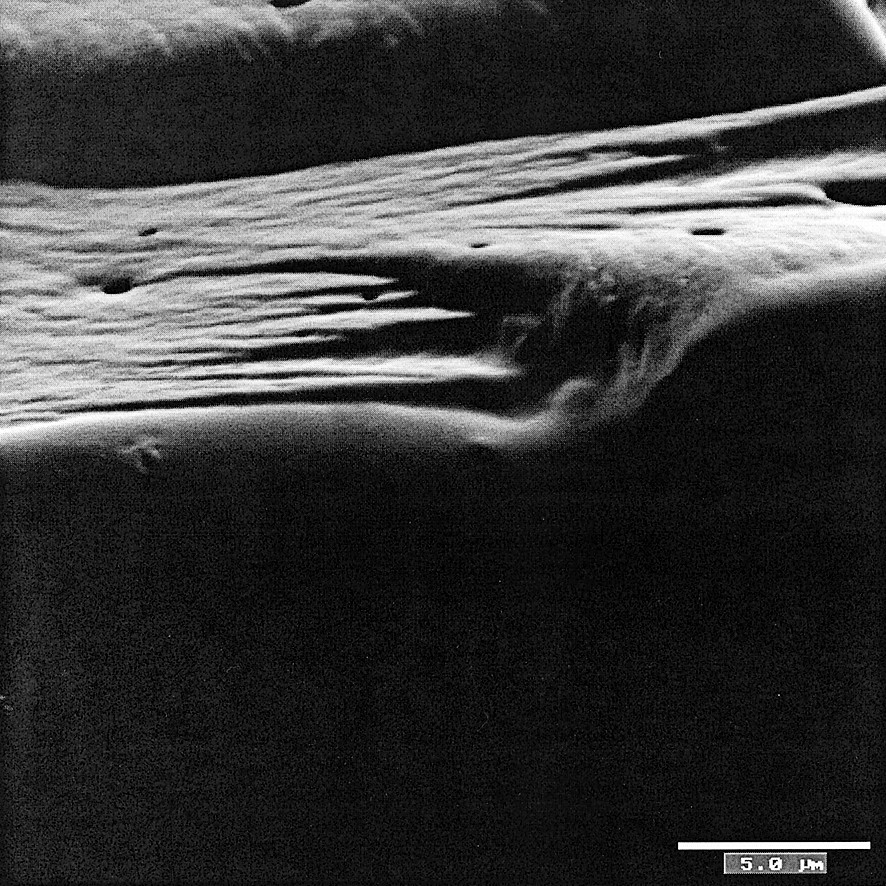
Scanning electron microscopy image of the Mn12 film covered with gold and palladium (voltage = 3 kV). The picture shows the border of the homogeneous film.
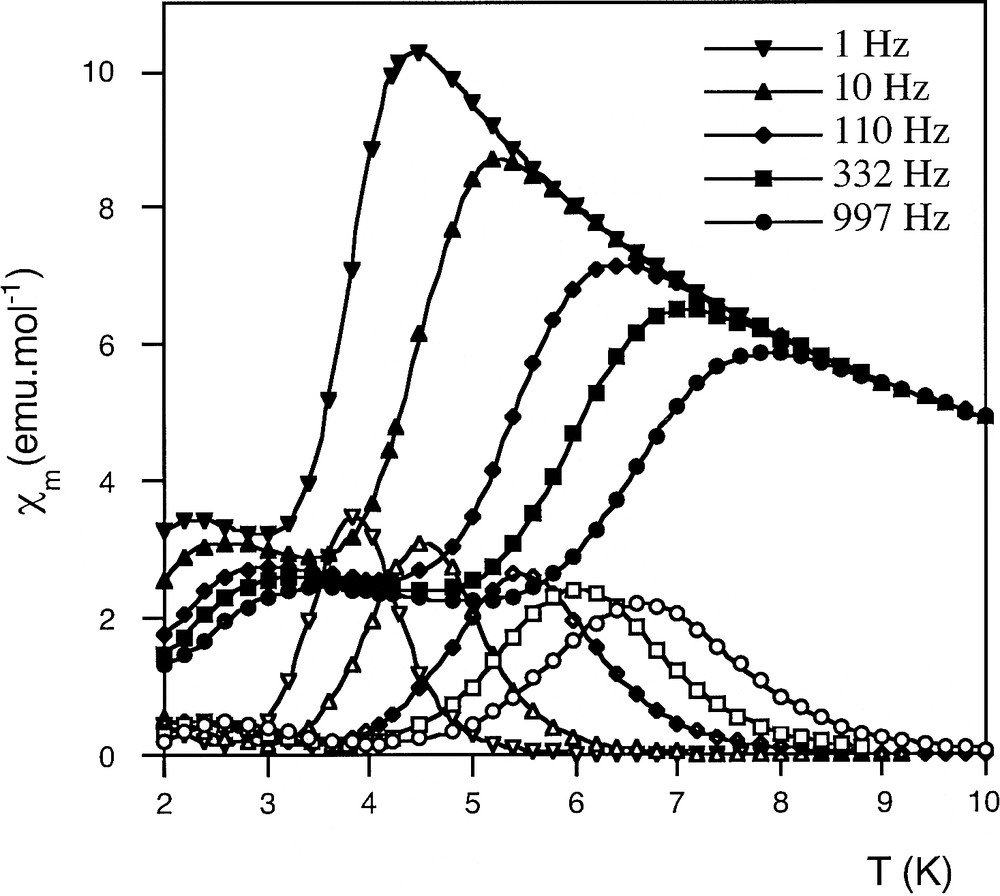
AC susceptibility versus T plot of the Mn12 film at various frequencies. Full and empty symbols correspond, respectively, to the in-phase and out-of-phase components of the AC magnetic susceptibility. The temperatures of the maxima of the out-of-phase susceptibility peaks were fitted to the Arrhenius equation to obtain an energy barrier, Ueff = 63 K, for the relaxation of the magnetization.

Magnetization hysteresis loop for the Mn12 film at 2 K.
In the second approach the possibility to deposit a monolayer of a cationic derivative of Mn12 on an anion-coated gold surface was explored. The cationic cluster employed in this study was (Mn12O12(OOCC6H5CH2N(nBu)3)16(H2O)4)(PF6)16 (Mn12Z), which has a 16+ charge [12]. Preliminary studies were performed on gold beads prepared by annealing the tip of a gold wire in a gas-oxygen flame. SAMs of thioctic acid, 1, and sodium 2-mercaptoethanesulfonate, 4 [10b, 13], (Fig. 7) were prepared by immersing glass-sealed gold beads electrodes for 24 h in an ETOH solution of 1 and 4 (1 mM and 10 mM respectively). Deprotonation of the thioctic acid monolayers was obtained by immersing these covered gold electrodes in a pH 8 borate buffer aqueous solution [14].
In order to adsorb the cationic Mn12Z derivative, these electrodes were then soaked in an organic solution containing variable concentrations of Mn12Z and a little amount of criptand in order to remove the alkaline cation easily. The procedure to study the formation of the second monolayer involves cyclic voltammetric experiments. However, in the present case, this method has shown to be insensitive to the formation of the SMM monolayer, due to the poor cyclic voltammetric response of Mn12Z. So, we should perform other measurements such as AFM to conclude. This work is in progress.
4 Conclusions
In this work, we have explored the possibilities to create layered organizations of inorganic magnetic clusters using the Langmuir–Blodgett technique or attaching the clusters on a metal surface. At present chemists are obtaining functional molecules that are being studied both in solution and in the solid state. More and more, there is a need to control the organization of these molecules in one-, two-, or three-dimensions in view of their potential applications. This step is just at the beginning. The two techniques reported in the present work are expected to play a very relevant role in this context in the near future.
Acknowledgements
This work has been supported by the European Union (network MOLNANOMAG), by the Spanish Ministry of Science and Technology (Grant MAT2001-3507) and by the Generalidad Valenciana (Grant GV01-312). M. C.-L and F. M. R. thank the Spanish Ministry of Science and Technology for a RyC contract. A. F.-A thanks the ‘Universidad de Valencia’ for a pre-doctoral fellowship.


