1 Introduction
In light of their unique molecular structure, dendrimers have attracted increasing attention in the past years [1]. With regard to the development of suitable procedures to synthesize monodispersed dendrimers, more and more emphasis is being placed on the design and study of functionalized dendrimers [1]. Of particular current interest is the use of dendritic architectures to mimic globular proteins owing to the ability of such macromolecules to surround active core units, thus creating specific site-isolated nano-environments capable of affecting dramatically the properties of the core moiety [2–3]. A variety of experimental techniques have been employed to provide evidence for the shielding of the core and to ascertain the effect of the surrounding dendrons. For example, kinetic studies of chemical reactions involving the central core unit give insight into substrate diffusion through the dendritic shell and allow for an evaluation of the accessibility of the core [2]. On the other hand, specific changes in the nano-environment of electroactive and/or photoactive cores are conveniently analysed by monitoring the redox and/or photophysical properties as a function of the generation number [2–5]. As a part of this research, we have prepared various series of dendrimers to study specific effects resulting from the attachment of dendrons on core molecules. These results are summarized in the present account to illustrate our current understanding of macromolecular encapsulation using dendrimers.
2 Dendrimers with an electroactive bis(phenanthroline) copper(I) core
Unambiguous structural characterization of large dendrimers by X-ray crystallography is nearly impossible; therefore, the only information about dendritic encapsulation is derived from the effect the dendritic shell has on some properties of the central core unit. To probe the relationship between dendrimer generation, structure, and core encapsulation, redox-core dendrimers have been extensively investigated [4]. A recent study involving dendrimers with a bis(phenanthroline)copper(I) core (Fig. 1) shows the typical effect of the dendritic environment on the electrochemical characteristics of the core [6].

Dendrimers with an electroactive bis(phenanthroline) copper(I) core.
Cyclic voltammetry of 1, performed at 100 mV s–1 in semi-infinite diffusion conditions in CH2Cl2/0.5 M n-Bu4PF6, exhibits a classical signal for a reversible one-electron process corresponding to the oxidation of the copper cation at 0.62 V vs. Fc+/Fc (Fig. 2). By increasing the size of the dendritic shell, the electron transfer kinetic is attenuated as judged by a decrease in the peak current and an increase in the peak potential differences (Fig. 2). The electron transfer kinetics of the highest generation compound 5 is estimated to be close to 5 × 10–4 cm s–1, compared to 5 × 10–3 cm s–1 for 1. The latter kinetic effect has been observed for several examples of dendrimers with an electroactive core [7–13] and illustrates the dendritic shell effect that leads to a more hindered approach of the core to the electrode.
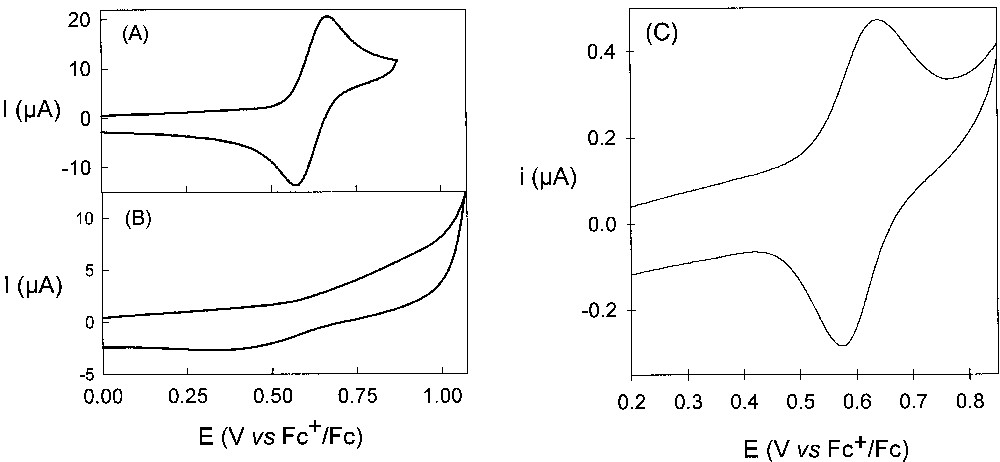
Left: cyclic voltammograms of 1 (A) and 5 (B) in CH2Cl2/n-Bu4PF6 (0.5 M) at 100 mV s–1. Right: voltammogram of 5 (C) in CH2Cl2/n-Bu4PF6 (0.5 M) at 2 mV s–1 in TLCV.
In some specific cases, changes of the nano-environment of electroactive cores can be analysed from the variation in the thermodynamic redox potential with the generation number [3–4]. However, the slow electron transfer kinetics observed for large electroactive dendrimers appear to be a severe limitation for the determination of their redox characteristics (the shielding of the central core is so effective in some cases that charge transfer could not be detected by classical CV measurements). Levillain has recently shown that the very weak electroactivity observed for the copper(I) bis-phenanthroline core of dendrimer 5 in classical CV can be enhanced by thin layer cyclic voltammetry (TLCV) [6]. Effectively, TLCV analysis of the copper(I) bis-phenanthroline core of the largest compound (5) at low scan rate (2 mV s–1) reveals a nice and well-resolved signal characteristic of a reversible one-electron process (Fig. 2). Indeed, the voltammograms are similar for compounds 1–5 under these experimental conditions. Therefore, TLCV is an efficient tool to determine the redox characteristics of large electroactive dendrimers.
3 A fullerene core to probe dendritic shielding effects
The synthesis of fullerene-functionalized dendrimers, i.e. fullerodendrimers, is currently an area of considerable interest [14–17]. In particular, the unusual chemical and physical properties of fullerene derivatives make fullerodendrimers attractive candidates for a variety of interesting features in supramolecular chemistry and materials science [18–22]. As part of this research, we have recently shown that dendrimers with a fullerene core are good candidates to demonstrate the shielding effects resulting from the presence of the surrounding dendritic shell [23–25]. Effectively, the lifetime of the first triplet excited state of fullerene derivatives is very sensitive to the solvent, and lifetime measurements in different solvents can be used to evaluate the degree of isolation of the central C60 moiety from external contacts. With this idea in mind, we have prepared fullerodendrimers 6–9 (Fig. 3). In the design of these compounds, it was decided to attach poly(aryl ether) dendritic branches terminated with peripheral triethyleneglycol chains to obtain derivatives soluble in a wide range of solvents [23].

Fullerodendrimers 6–9.
The photophysical properties of 6–9 have been systematically studied in different solvents (PhMe, CH2Cl2 and CH3CN) [24]. Interestingly, a steady increase of triplet lifetimes in air-equilibrated solutions is found by increasing the dendrimers size in all solvents (Table 1), suggesting that the dendritic wedges are able to shield, at least partially, the fullerene core from external contacts with the solvent and from quenchers such as molecular oxygen. The increase is particularly marked in polar CH3CN, where a better shielding of the fullerene chromophore is expected as a consequence of a tighter contact between the strongly non-polar fullerene unit and the external dendritic wedges; in this case a 45% lifetime prolongation is found in passing from 6 to 9 (23% and 28% only for PhCH3 and CH2Cl2, respectively). In other words, the degree of encapsulation varies with the solvent. It must be also emphasized that the triplet lifetimes of 9 in the three solvents are rather different from each other, likely reflecting specific solvent-fullerene interactions that affect excited state deactivation rates. This suggests that, albeit a dendritic effect is evidenced, even the largest wedge is not able to provide a complete shielding of the central fulleropyrrolidine core in 9 [24]. The latter hypothesis was confirmed by computational studies. As shown in Fig. 4, the calculated structure of 9 revealed that the dendritic shell is unable to completely cover the fullerene core (it must be noted that the calculations have been performed in the absence of solvent, our aim being only to estimate the possible degree of isolation).
Lifetime of the first triplet excited state of 6–9 in air-equilibrated solutions, determined by transient absorption at room temperature [24]
| Compound | τ (ns) in PhMe | τ (ns) in CH2Cl2 | τ (ns) in CH3CN |
| 6 | 279 | 598 | [a] |
| 7 | 304 | 643 | 330 |
| 8 | 318 | 732 | 412 |
| 9 | 374 | 827 | 605 |

Calculated structure of fullerodendrimer 9 [25].
Note. The Molecular Dynamics studies have been performed on SGI Origin 200 and Octane2 workstations using the Discover 3 software from Accelrys (www.accelrys.com) with the pcff force field. The previously minimized structures were allowed to equilibrate for 500 ps at a 300 K isotherm by the MD simulation (in the NVT ensemble with a time step of 1 fs).
More recently, we have reported a new series of dendrimers with a fullerene core (Fig. 5) [25]. When compared to compounds 6–9, it was decided to attach two dendritic branches to the C60 unit in order to improve the shielding of the central chromophore. The photophysical properties of 10–13 have been investigated in different solvents (PhMe, CH2Cl2 and CH3CN) and, as observed for 6–9, a steady prolongation of air-equilibrated triplet lifetimes is found for 10–13 by increasing the dendrimer’s size in all solvents (Table 2).

Fullerodendrimers 10–13.
Lifetime of the first triplet excited state of 10–13 in air-equilibrated solutions determined by transient absorption at room temperature [25]
| Compound | τ (ns) in PhMe | τ (ns) in CH2Cl2 | τ (ns) in CH3CN |
| 10 | 288 | 611 | 314 |
| 11 | 317 | 742 | 380 |
| 12 | 448 | 873 | 581 |
| 13 | 877 | 1103 | 1068 |
By inspecting the data of air-equilibrated triplet lifetimes in Table 2, some trends can be emphasized. For instance, although the lifetime of 10 is substantially different in CH2Cl2 and CH3CN (611 and 314 ns, respectively) an identical lifetime, within experimental uncertainties, is measured for 13 (≈ 1100 ns). This suggests that in CH2Cl2 and CH3CN the fullerene core of 13 is buried inside the dendritic cage, and has negligible interactions with the solvent molecules. Notably, in toluene and CH3CN, the lifetime of 10 is shorter than in CH2Cl2, but in both solvents an increase of over 200% is observed for 13 and the triplet lifetimes of the largest compound tend towards a similar value suggesting that the fullerene core is in a similar environment whatever the nature of the solvent is. In other words, the C60 core is, to a large extent, not surrounded by solvent molecules but substantially buried in the middle of the dendritic structure. The latter hypothesis is quite reasonable based on the calculated structure of 13 (Fig. 6), showing that the dendritic branches are able to fully cover the central fullerene core.

Calculated structure of fullerodendrimer 13 [25].
The dendritic effect evidenced for 6–9 and 10–13 might be useful to optimise the optical limiting properties characteristic of fullerene derivatives. Effectively, the intensity dependant absorption of fullerenes originates from larger absorption cross sections of excited states compared to that of the ground state [26], therefore the increased triplet lifetime observed for the largest fullerodendrimers may allow for an effective limitation on a longer time scale. For practical applications, the use of solid devices is largely preferred to solutions and inclusion of fullerene derivatives in sol–gel glasses has shown interesting perspectives [27]. However, faster de-excitation dynamics and reduced triplet yields are typically observed for fullerene-doped sol-gel glasses when compared to solutions [28]. The latter observations are mainly explained by two factors: (i) perturbation of the molecular energy levels due to the interactions with the sol-gel matrix and (ii) interactions between neighbouring fullerene spheres due to aggregation [28]. Therefore, the encapsulation of the C60 core evidenced by the photophysical studies for both series of fullerodendrimers might also be useful to prevent such undesirable effects. The incorporation of fullerodendrimers 6–13 in sol–gel glasses has been easily achieved by soaking mesoporous silica glasses with a solution of 6–13 [24]. For the largest compounds, the resulting samples only contain well-dispersed fullerodendrimer molecules. Preliminary measurements on the resulting doped samples have revealed efficient optical limiting properties [24]. For example, the optical transmission as a function of the fluence of the laser pulses is shown in Fig. 7 for a sol–gel sample containing compound 9. The transmission remains nearly constant for fluences lower than 5 mJ cm–2. When the intensity increases above this threshold, the effect of induced absorption appears, and the transmission diminishes rapidly, thus showing the potential of these materials for optical limiting applications. Further studies are underway in order to determine the influence of the dendritic branches on the optical limiting behaviour of these composite materials.
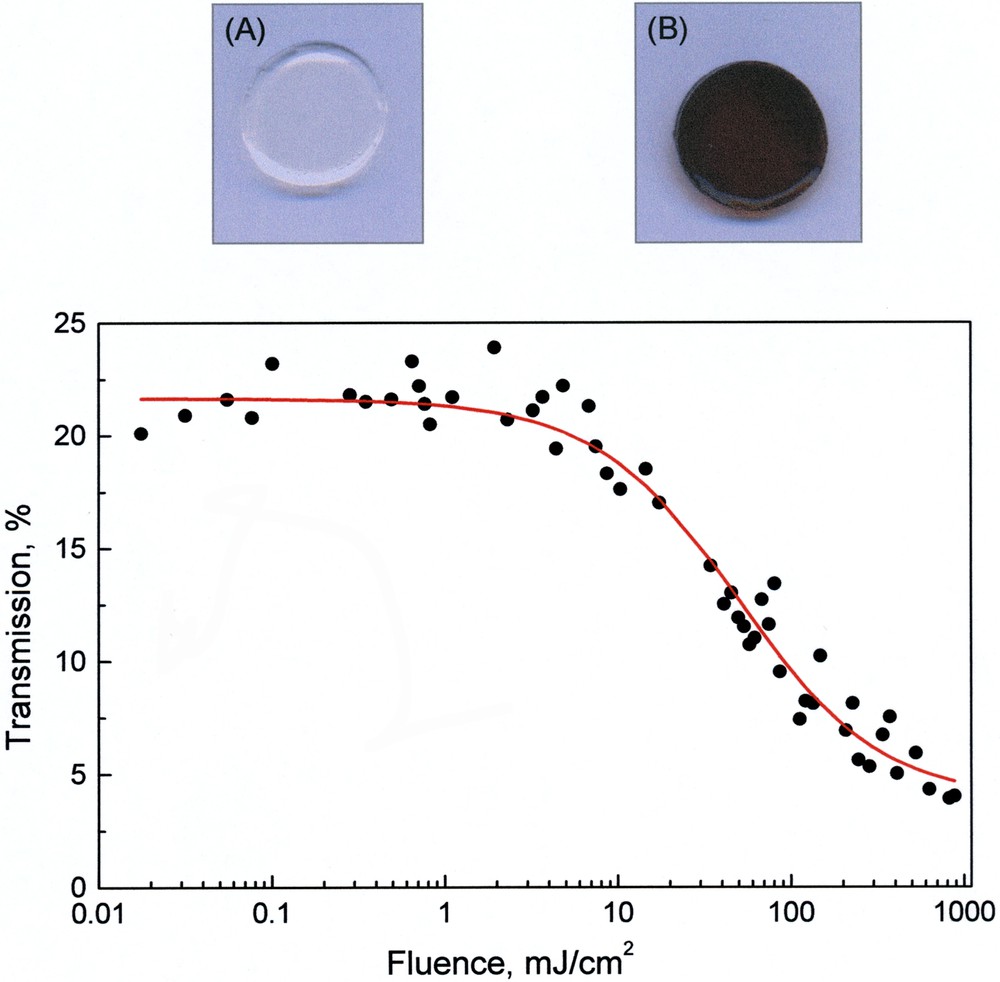
Mesoporous sol–gel glass before (A) and after (B) soaking with a solution of 9 and transmission versus incident fluence at 532 nm of the sol–gel sample containing dendrimer 9.
4 Dendrimers with a cyclotriveratrylene core
In light of their multifunctionality and specific shape, dendrimers have been regarded as attractive candidates for applications in host–guest chemistry [1]. Functionalization of the dendritic surface or branches with recognition sites appears as a possible approach to dendrimers with defined binding properties. In such cases, an enhanced efficiency through simultaneous association with several substrates has been observed [29, 30]. Alternatively, the central core of the dendrimer can be a cyclophane with well-defined complexation ability. Diederich et al. have described examples of such dendritic host molecules they called dendrophanes [31–33]. The recognition site is, at least to some extent, shielded from its medium, implying a typical nano-environment inside the dendrimer and such structures can serve as models for globular proteins. As part of this research, we have shown that the binding properties of a dendrophane can be modulated by the size or the polarity of the surrounding dendrons [34, 35]. More specifically, we found that the association constants for the binding of fullerene derivatives with the dendritic cyclotriveratrylene (CTV) derivatives 14–17 (Fig. 8) in CH2Cl2 or C6H6 are significantly increased as the generation number of the dendritic substituents is increased.

Dendrophanes 14–17 and association constants for the binding of C60 determined by UV/Vis titrations in CH2Cl2 at 298 K [34].
Even if the shape and the dimension of the CTV macrocycle appears well predetermined for the complexation of the fullerene sphere, the binding constant for C60 found in organic solvents with CTV derivatives is generally low. This could be due to the fact that only a small part of the C60 surface is actually in contact with the cavity of the macrocyclic CTV receptor. As mentioned above, the binding studies of 14–17 reveal that the surrounding dendritic branches are able to increase the inclusion abilities of the CTV central core for fullerenes. This effect could be attributed to additional electronic donor-acceptor π–π interactions between the polyaryl ether dendrons and the fullerene guest. However, we also believe that the additional phenyl subunits are not the sole explanation for the observed effect; the dendritic structure itself must play an important role for the binding of C60. Actually, the functionalization of the CTV core with polybenzyl ether dendrons is able to generate an internal cavity with more appropriate shape and dimension for interactions with the fullerene guest. This effect is more pronounced as the size of the dendritic substituents is increased. As a result, the Ka values increase when the surrounding dendrons become larger.
5 Conclusion
Specific properties of several series of dendrimers have been systematically investigated as a function of the generation number. The encapsulation of a redox-active unit, namely a bis(phenanthroline) copper(I) complex, has been evidenced by the attenuation of the electron transfer rate with increasing molecular size. We have also shown that fullerene derivatives are excellent probes for evidencing dendritic shielding effects. In particular, the triplet lifetimes of a C60 core can be used to evaluate its degree of isolation from external contacts. In addition, the protective effect observed for fullerodendrimers 9 and 13 might be useful for optical limiting applications. Finally, the inclusion abilities of dendrophanes with a CTV core for fullerenes have shown that the dendritic architecture is not only able to isolate a central functional core but can also modulate its binding properties by means of the size and the nature of the surrounding dendrons. All our findings point out that the effect dendritic wedges have on the properties of encapsulated functional groups allows the establishment of structure-property relationships enabling us to gain more insight into the crucial role of site isolation, which will ultimately lead to a better understanding of biological systems. Effectively, the ability of proteins to generate specific nano-environments around active centres and catalytic sites is at the origin of their unique properties.
Acknowledgements
This work was supported by the CNRS and the French Ministry of Research (ACI Jeunes Chercheurs). I would like to warmly thank all my co-workers and collaborators for their outstanding contributions, their names are cited in the references.


