1 Introduction
Liquid-crystalline dendrimers have generated enthusiastic studies at the frontiers of chemistry, materials science and biology [1, 2]. The association of a well-defined macromolecular structure with the features of liquid crystals represents an ideal combination for the design of sophisticated supramolecular architectures that are capable to bring fundamental information to better our understanding of complex natural-occurring phenomena [3] and for the elaboration of novel materials for applications in nanotechnology.
A great variety of mesomorphic dendrimers have been reported, and all types of mesophases have been observed [4]. Of particular interest is the possible dependence of the nature of the mesophases with the dendrimer generation. Such a structure–property relationship offers the unique opportunity to tune the liquid-crystalline behaviour with the size of the dendrimer.
With the view to develop new organometallic liquid crystals, we undertook the design, synthesis and study of ferrocene-containing thermotropic dendrimers. Owing to the presence of the ferrocene unit, such materials are of interest for the elaboration of dendritic switches, as reported for low molar-mass liquid crystals [5, 6] and side-chain liquid-crystalline polymers [7], and anisotropic molecular batteries [8].
The design of ferrocene-containing liquid-crystalline dendrimers with tailor-made properties is a difficult problem: (i) the properties associated with ferrocene should depend on its location within the dendrimer (in the core, at the branching points, at the periphery), and (ii) the type of the liquid-crystalline phases is connected to the dendrimer (generation), and to the mesogenic groups (structure, number, location). Obviously, the study of a large series of compounds is required to reach the point where the influence of all parameters can be controlled.
In this report, we present two second-generation dendrimers (Fig. 1) that differentiate by the position of the ferrocene unit and by the liquid-crystalline promoter: in 1a, the ferrocene is located near the branching point and cholesterol acts as liquid-crystalline promoter, whereas in 1b, ferrocene is located at the periphery and the liquid-crystalline segment is a rod containing four aromatic rings.

Ferrocene-containing thermotropic liquid-crystalline dendrimers.
2 Synthesis
The synthetic strategy applied to prepare 1a,b is based on the convergent methodology [9, 10] illustrated in Fig. 2. The ferrocene unit was introduced at an early stage to obtain first-generation dendrons 2a,b. Removal of the benzyl protected group under hydrogenation conditions gave 3a,b. Condensation of 3a,b with 4 (used to synthesize 2a,b) furnished second-generation dendrons 5a,b. Deprotection of 5a,b under hydrogenation conditions yielded 6a,b. Finally, esterification of 6a,b with 1,3,5-benzenetricarbonyltrichloride (7) led to the targeted dendrimers 1a,b [11, 12].
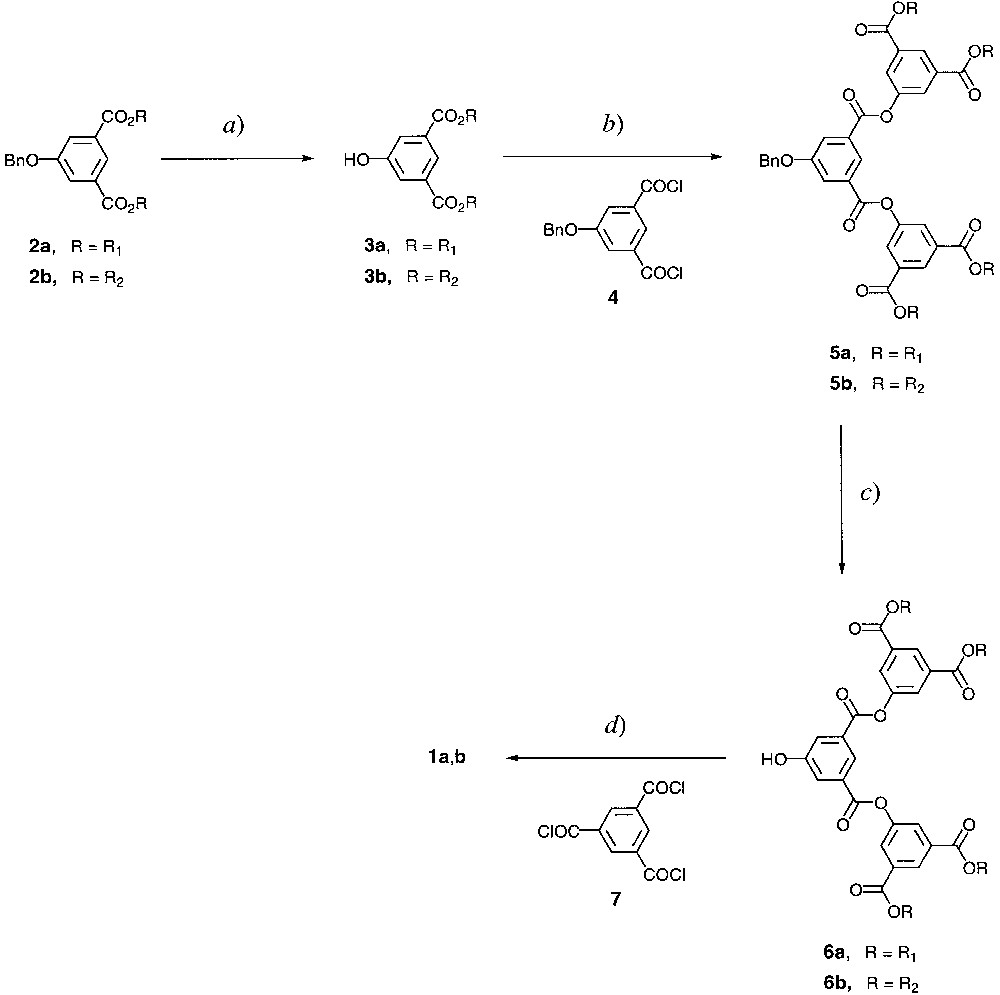
Reagents and conditions. For 1a: (a) H2, Pd/C, CH2Cl2, 8 h, 94%; (b) triethylamine (Et3N), CH2Cl2, reflux, overnight, 63%; (c) H2, Pd/C, CH2Cl2, overnight, 89%; (d) Et3N, CH2Cl2, reflux, overnight, 84%. For 1b: (a) H2, Pd/C, CH2Cl2/EtOH, 7 h, 91%; (b) Et3N, CH2Cl2, reflux, overnight, 90%; (c) H2, Pd/C, CH2Cl2/EtOH, room temperature, overnight, 73%; d) Et3N, CH2Cl2, reflux, 21 h, 88%. For R1 and R2, see Fig. 1.
3 Liquid-crystalline properties
The liquid-crystalline properties of 1a,b were investigated by polarized optical microscopy (POM), differential scanning calorimetry (DSC), and X-ray diffraction (XRD). Both dendrimers are thermally stable (no decomposition was detected by POM or DSC). The phase transition temperatures are reported in Table 1. Dendrimers 1a,b displayed clear liquid-crystalline behaviour. They both gave rise to enantiotropic smectic phases. For 1a, the mesophase could be identified by POM as a smectic A phase from the formation of focal-conic and homeotropic textures. In case of 1b, no typical texture could be obtained by POM. The smectic nature of the mesophase (smectic A or smectic C) was established by XRD. Note that 1b shows a much higher isotropization temperature than 1a. The structure of the mesophases can be explained by the formation of layers containing the dendrimer cores with interdigitation of the mesogenic units from layer to layer. Both compounds gave similar d layer spacings (for 1a: d = 44.1 Å at 105 °C and for 1b: d = 47 Å at 140 °C). The mesomorphic behaviour of 1a,b is consistent with their structures.
Phase-transition temperatures of 1a and 1b
| Compound | Transitiona | Temperature (°C) |
| 1a | Tg | 52 |
| SA → I | 169 | |
| 1b | Cr → S X | 119 |
| SX → I | 256 |
Despite the fact that 1a,b have the same dendrimer generation (i.e. same number of ferrocene and mesogenic units), direct comparison of their properties is not straightforward because of their structural differences (substitution and location of the ferrocene units; nature and location of the liquid-crystalline promoters). Further compounds are required to establish a structure–property relationship. However, the examples reported herein indicate that ferrocene-containing liquid-crystalline dendrimers can be designed with no limitation regarding the location of the ferrocene units. This is of interest for specific applications of ferrocene-based materials.
4 Conclusion
We have developed synthetic tools for the preparation of liquid-crystalline metallodendrimers. Our strategy is not limited to ferrocene, but other organometallics can be used. Regarding the fascinating results reported for ferrocene-based dendrimers [13–16], the incorporation of ferrocene or other organometallic units within ordered structures capable to self-assemble into mesomorphic organizations could lead to polyfunctional materials exhibiting novel properties and functions.



Vous devez vous connecter pour continuer.
S'authentifier