1 Introduction
The enantioselective addition of organometallics to aldehydes and imine derivatives is a powerful method for the asymmetric synthesis of chiral alcohols and amines [1, 2]. Within this technique, the catalytic enantioselective addition of dialkylzincs is one of the most important methods [3−5]. Following our finding that chiral β-amino alcohol accelerates the addition of diethylzinc to benzaldehyde [6], enantioselective addition of dialkylzincs to aldehydes using β-amino alcohols as chiral catalysts has been developed [3–5]. We have devised highly enantioselective catalysts that include diphenyl(1-methylpyrrolidin-2-yl)methanol (DPMPM) [7], and N,N-dialkylnorephedrines such as N,N-dibutylnorephedrine (DBNE), etc. [8, 9]. The chiral amino alcohols act as Lewis base in activation of dialkylzincs; enabling the enantioselective addition of dialkylzincs to aldehydes (Fig. 1). Thus, (1S, 2R)-DBNE catalyses the enantioselective formation of sec–alcohols with S configuration (when the priority order is R1>R2). On the other hand, (1R, 2S)-DBNE produces (R)–alcohols.

Moreover, we have reported that N,N-dialkylnorephedrines promote the enantioselective addition of dialkylzincs to N-diphenylphosphinylimines to produce enantiomerically enriched N-diphenylphosphinylamines with high ee. Because the N-diphenylphosphinyl group is easily removed by acid hydrolysis, the overall process is a convenient method for the preparation of chiral amines [10−16].
In the course of our continuing study on enantioselective synthesis using polymer- [17−19] and silica- [20] bound heterogeneous chiral catalysts, we became interested in the attractive characteristics of dendrimers. Dendrimers, orderly hyperbranched macromolecules, are defined as polymers with a particular molecular weight and molecular architecture. All of the dendritic branches are capable of adding chiral functionalities to their chain-ends. Due to the well-defined molecular weight and the molecular architecture with its regularly branched structure, nearly all of the chiral sites at the periphery would work effectively in approximately the same chiral circumstances. Although several chiral ligands with dendritic substituent(s) in asymmetric synthesis have been used in asymmetric synthesis [21−28], chiral dendrimers with multiple chiral sites at the periphery have attracted less attention [29−33]. We describe here our design of chiral dendritic catalysts and ligands, and their application in enantioselective synthesis.
2 Results and discussion
2.1 Chiral amino alcohols bound to diamine, diimine and polyamidoamine (PAMAM) dendrimers as chiral ligands for the enantioselective addition of diethylzinc to N-diphenylphosphinylimines
We prepared dendritic chiral ligands by attaching ephedrine derivatives at the periphery of polyamidoamine (PAMAM) dendrimers. Dendritic chiral ligands 3 and 4 bear four and eight sites of chiral amino alcohols, respectively (Fig. 2). We also prepared chiral diamine 1 and diimine 2 possessing ephedrine moieties.
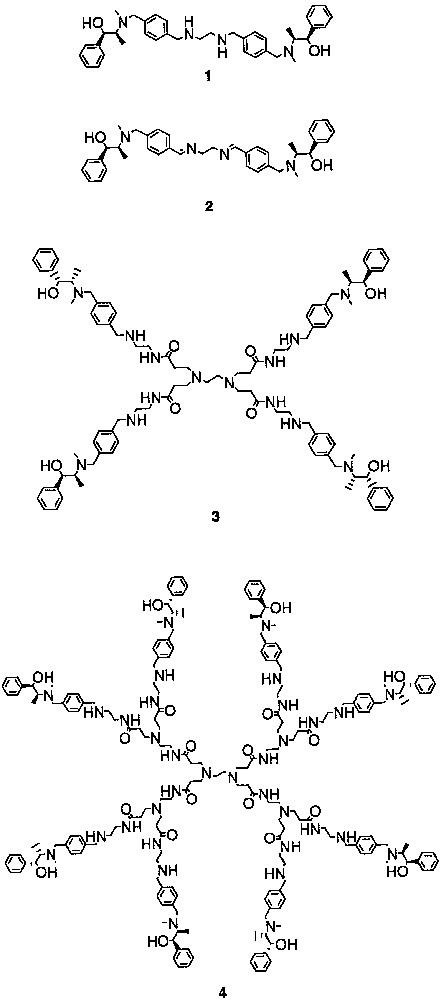
Chiral diamine 1, diimine 2 and dendrimers 3 and 4 were found to act as chiral ligands in the enantioselective addition of diethylzinc to N-diphenylphosphinylimines (Eq. (2)) [34]. Reaction in the presence of chiral diamine 1 and diimine 2 gave (R)-N-diphenylphosphinylamine with 92% ee. However, according to the increase in the size of PAMAM-based chiral dendrimers 3 and 4, the enantioselectivity of the reaction decreased, and N-diphenylphosphinylimines with only moderate ee were obtained. Coordination of nitrogen and oxygen atoms of the PAMAM skeleton to the zinc presumably resulted in the need for an excess amount of diethylzinc, the change of the conformation of chiral dendrimers, and the subsequent decrease in enantioselectivity.
2.2 Chiral dendritic catalysts and ligands with hydrocarbon [poly(phenylethyne)] backbones
In order to attain high enantioselectivity by using a chiral dendritic catalyst and ligand, it was necessary to avoid unfavorable coordination between the dialkylzinc reagent and the framework of the dendrimer. Thus, we devised chiral dendrimers 5 and 6 with hydrocarbon [poly(phenylethyne)], i.e., without heteroatoms, with a backbone bearing three and six chiral ephedrine derivatives at the periphery, respectively (Fig. 3) [35]. In addition, each chiral site of the dendritic catalysts and ligands 5 and 6 is expected to work independently of other chiral sites because of the relatively rigid phenylethyne and approximately planar structure of the backbone.
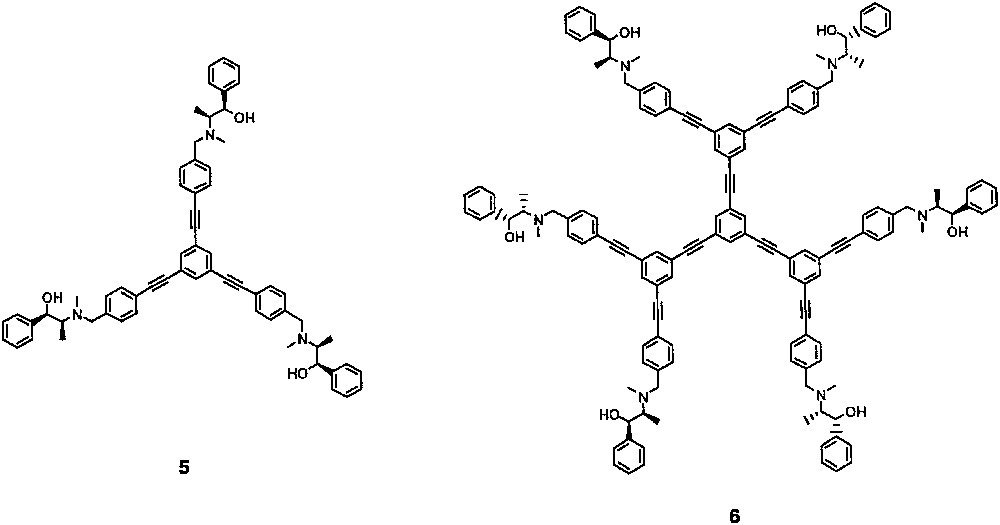
Catalytic enantioselective addition of dialkylzincs to aldehydes using these chiral dendritic catalysts 5 and 6 gave enantiomerically enriched sec–alcohols with 77−86% ee (Eq. (1)). The results are shown in Table 1. Enantioselective addition of diisopropylzinc to benzaldehyde 7a in the presence of 3.3 mol% of chiral dendritic catalyst 5, bearing three chiral ephedrine moieties, gave (R)-2-methyl-1-phenylpropan-1-ol 8a with 86% ee in an isolated yield of 63% (entry 1). The generality of dialkylzinc and aryl aldehydes is also shown (entries 2−4). Chiral dendritic catalyst 6 bearing six chiral sites catalyzed the addition of diisopropylzinc to aldehydes with high (80−86%) enantioselectivities (entries 5 and 6). Thus, both dendritic chiral catalysts 5 and 6 act as highly enantioselective catalysts in the addition of dialkylzincs to aldehydes.
Enantioselective addition of dialkylzincs to aldehydes using chiral dendritic catalysts 5 and 6
| Aldehyde | R2 | Chiral catalyst | (R)–Alcohol | ||||
| Entrya | R1 | yield (%) | eeb (%) | ||||
| 1 | phenyl | 7a | i-Pr | 5 | 8a | 63 | 86 |
| 2 | phenyl | 7a | Et | 9a | 61 | 78 | |
| 3 | 2-naphthyl | 7b | i-Pr | 8b | 59 | 84 | |
| 4 | p-tolyl | 7c | i-Pr | 8c | 67 | 77 | |
| 5 | phenyl | 7a | i-Pr | 6 | 8a | 70 | 80 |
| 6 | 2-naphthyl | 7b | i-Pr | 8b | 32 | 86 |
Next, we examined the enantioselective addition of diethylzinc to N-diphenylphosphinylimines using dendritic chiral ligands 5 and 6 (Table 2, Eq. (2)) [36]. Chiral dendrimer 5 (0.34 mol equiv) promotes the highly enantioselective addition of diethylzinc to N-diphenylphosphinylimines 10a−d to produce enantiomerically enriched (R)-N-diphenylphosphinylamines 11a−d with 71−94% ee in 73−80% yields (entries 1−4). Chiral dendrimer 6 (0.17 mol. equiv.) of a higher-order generation also accelerates the reaction to give enantiomerically enriched (R)-N-diphenylphosphinylamines 11a−c with 85−90% ee in yields of 74−79% (entries 5−7). Thus, these chiral dendritic ligands 5 and 6 were found to be highly enantioselective chiral ligands for the enantioselective addition of dialkylzincs to N-diphenylphosphinylimines. From our previous study, it is known that a stoichiometric amount of chiral β-amino alcohol is required to assure high yield in the enantioselective addition of dialkylzinc to N-diphenylphosphinylimines [13,14]. Because the number of chiral sites at the periphery of ligand 5 (0.34 mol. equiv.) bearing three chiral sites (0.34 × 3 = 1.0) and 6 (0.17 mol equiv) bearing six chiral sites (0.17 × 6 = 1.0) is equimolar against aldehydes, the high yields and ees of N-diphenylphosphinylimines attained by using dendritic ligands 5 and 6 suggest that nearly all of the chiral sites at the periphery work effectively.
Enantioselective addition of diethylzinc to various N-diphenylphosphinylimines using chiral dendritic ligands 5 and 6
| N-diphenylphosphinylimine | chiral ligand | (R)-N-diphenylphosphinylamine | |||||||
| entrya | R1 | mol. equiv. | yield (%) | eeb (%) | |||||
| 1 | phenyl | 10a | 5 | 0.34 | 11a | 73 | 89 | ||
| 2 | p-tolyl | 10b | 11b | 77 | 94 | ||||
| 3 | 2-naphthyl | 10c | 11c | 80 | 89 | ||||
| 4 | 2-furyl | 10d | 11d | 77 | 71 | ||||
| 5 | phenyl | 10a | 6 | 0.17 | 11a | 77 | 87 | ||
| 9 | p-tolyl | 10b | 11b | 79 | 90 | ||||
| 10 | 2-naphtyl | 10c | 11c | 74 | 85 |
2.3 Chiral dendritic catalysts and ligands with flexible carbosilane backbones
In the preceding sections, we described chiral dendrimers 3 and 4 with a flexible backbone capable of coordinating to dialkylzinc, and chiral dendrimers 5 and 6 with a relatively rigid backbone with less capability of coordinating to dialkylzinc. Then, what is the enantioselectivity of chiral dendrimers with a flexible backbone that have less capability of coordinating to dialkylzinc?
In this section, we describe the preparation of chiral dendritic catalysts and ligands with a poly(carbosilane) backbone bearing chiral ephedrine sites at the periphery (Fig. 4). The backbone of poly(carbosilane) was reported by van Koten et al. in the nickel catalyzed Kharash addition of polyhalogenoalkanes to alkenes [37]. We became interested in preparing chiral dendritic catalysts and ligands with poly(carbosilane) backbones bearing chiral sites at the periphery, and in applying these chiral dendritic catalysts and ligands to the enantioselective addition of dialkylzincs to aldehydes and N-diphenylphosphinylimines. The carbosilane backbone is more flexible than the poly(phenylethyne) backbone, and the backbone hardly coordinates to dialkylzinc reagents.

We synthesized chiral dendrimers 12 and 13 bearing four and 12 chiral ephedrine sites, respectively. Chiral dimer 14 was also prepared. These chiral catalysts were employed in the enantioselective addition of dialkylzincs to aldehydes (Eq. (1)) [38]. The results are shown in Table 3. In the presence of 5 mol% of chiral dendritic catalyst 12, enantioselective addition of diisopropylzinc to 2-naphthaldehyde 7b produces (R)-2-methyl-1-naphthylpropan-1-ol 8b with 88% ee in 75% yield (entry 3). In a similar manner, enantioselective addition of diethylzinc and diisopropylzinc to aldehydes 7a, d, e and f using 12 as a chiral catalyst gave the corresponding enantiomerically enriched sec-alcohols with 82−93% ee (entries 1, 2 and 4−6). The ee reached 93% in the addition of diisopropylzinc to 3–phenylpropanal (entry 6). When chiral dendritic catalyst 13 (1.7 mol%) bearing 12 chiral sites was employed, enantiomerically enriched sec-alcohols 8a,b and d−f with 83−93% ees were obtained (entries 7−13). The highest, 93% ee, using catalyst 13 was attained in the enantioselective addition of diisopropylzinc to 3-phenylpropanal (entry 13). Chiral dendritic catalyst 13 can be recovered and used without any loss of reactivity and enantioselectivity (entries 7 and 8). It should be noted that the enantioselectivities attained by using chiral dendritic catalysts 12 and 13 are comparable with those attained by using chiral dimer catalyst 14 (Fig. 4).
Highly enantioselective addition of dialkylzincs to aldehydes using chiral dendritic catalysts 12, 13 or chiral dimer 14
| aldehyde | chiral catalyst | (R)–alcohol | |||||||
| entrya | R1 | R2 | (mol%) | yield (%) | ee (%)b | ||||
| 1 | phenyl | 7a | Et | 12 | (5.0) | 9a | 80 | 82 | |
| 2 | phenyl | 7a | i-Pr | 8a | 80 | 82 | |||
| 3 | 2-naphthyl | 7b | i-Pr | 8b | 75 | 88 | |||
| 4 | 1-naphthyl | 7d | i-Pr | 8d | 42 | 86 | |||
| 5 | 4-CH3OC6H4 | 7e | i-Pr | 8e | 80 | 85 | |||
| 6 | PhCH2CH2 | 7f | i-Pr | 8f | 56 | 93 | |||
| 7 | phenyl | 7a | i-Pr | 13 | (1.7) | 8a | 83 | 83 | |
| 8c | phenyl | 7a | i-Pr | 8a | 79 | 85 | |||
| 9 | phenyl | 7a | i-Pr | (5.0) | 8a | 84 | 86 | ||
| 10 | 2-naphthyl | 7b | i-Pr | (1.7) | 8b | 77 | 84 | ||
| 11 | 1-naphthyl | 7d | i-Pr | 8d | 42 | 87 | |||
| 12 | 4-CH3OC6H4 | 7e | i-Pr | 8e | 79 | 83 | |||
| 13 | PhCH2CH2 | 7f | i-Pr | 8f | 55 | 93 | |||
| 14 | phenyl | 7a | Et | 14 | (5.0) | 9a | 80 | 74 | |
| 15 | phenyl | 7a | i-Pr | 8a | 77 | 79 | |||
| 16 | 1-naphthyl | 7d | i-Pr | 8d | 42 | 88 |
Next, the enantioselective addition of diethylzinc to N-diphenylphosphinylimines was examined (Eq. (2)) [39]. The results are shown in Table 4. As expected, the enantioselective addition of diethylzinc to N-diphenylphosphinylimine 10c, promoted by chiral carbosilane dendrimer 12 (0.25 mol equiv), gave (R)-N-diphenylphosphinylimine 10c with high (92%) ee (entry 1). Similarly, the addition of diethylzinc to N-diphenylphosphinylimine 10c using a chiral dendritic ligand of a higher generation 13 (0.13 mol equiv) produced (R)-N-diphenylphosphinylimine 11c with 92% ee in 70% yield (entry 7). The use of a lesser amount (0.083 mol. equiv.) of 13 also produced imine 11c with 90% ee in 70% yield (entry 8). Chiral ligand 12 was recovered and reused without any loss of reactivity and enantioselectivity (entries 1 and 2). Diisopropylzinc can also be used (entries 4 and 11). The enantioselectivities of chiral dendritic ligands 12 and 13 are comparable with those of chiral dimer 14.
Highly enantioselective addition of dialkylzincs to N-diphenylphosphinylimines using chiral dendritic ligands 12, 13 or chiral dimer 14
| N-diphenylphosphinylimine | chiral ligand | N-diphenylphosphinylamine | |||||||
| entrya | R1 | R2 | (mol. equiv.) | yield (%) | ee (%)b | ||||
| 1 | 2-naphthyl | 10c | Et | 12 | (0.25) | 11c | 78 | 92 | |
| 2c | 2-naphthyl | 10c | Et | 11c | 77 | 91 | |||
| 3 | p-tolyl | 10b | Et | 11b | 71 | 90 | |||
| 4 | phenyl | 10a | iPr | 15 | 79 | 89 | |||
| 5 | phenyl | 10a | Et | 11a | 81 | 82 | |||
| 6 | 4-ClC6H4 | 10e | Et | 11e | 72 | 88 | |||
| 7 | 2-naphthyl | 10c | Et | 13 | (0.13) | 11c | 70 | 92 | |
| 8 | 2-naphthyl | 10c | Et | (0.083) | 11c | 70 | 90 | ||
| 9 | p-tolyl | 10b | Et | (0.13) | 11b | 71 | 84 | ||
| 10 | 4-ClC6H4 | 10e | Et | 11e | 74 | 85 | |||
| 11 | phenyl | 10a | i Pr | 15 | 70 | 86 | |||
| 12 | 2-naphthyl | 10c | Et | 14 | (0.50) | 11c | 71 | 94 | |
| 13 | phenyl | 10a | i Pr | 15 | 81 | 90 | |||
| 14 | phenyl | 10a | Et | 11a | 80 | 86 |
3 Summary
As described, we have developed chirally end-capped dendrimers with polyamidoamine, poly(phenylethyne) and poly(carbosilane) backbones as chiral catalysts and ligands for the enantioselective addition of dialkylzincs to aldehydes and N-diphenylphosphinylimines. Chiral dendrimers with poly(phenylethyne) and poly(carbosilane) backbones exhibit high enantioselectivity. We believe that the results reported here provide guidelines for the design of future dendritic chiral catalysts and ligands for enantioselective asymmetric synthesis.


