1 Introduction
The preparation of hydrophilic and stimuli responsive polymer latexes has attracted considerable and increasing attention as can be evidenced from the flourishing number of published works. In the case of thermally-sensitive microgel particles, earliest works relied on the precipitation polymerization in water of N-isopropylacrylamide (NIPAM) using N,N-methylenebisacrylamide (MBA) as a crosslinker as pioneered by Pelton et al. [1] and then by Kawaguchi et al. [2]. The precipitation polymerization rate of NIPAM monomer was reported to be rapid and the final formed particles exhibited a volume-phase-transition temperature (TVPT) over a broad range of temperature around the LCST of linear homopoly(NIPAM). Numerous works have been devoted to the preparation, characterization and application of various poly(NIPAM)-based particles [3,4], whereas, only a few ones dealt with thermally-microgel particles exhibiting higher TVPT than 32 °C such as poly(N-isopropylmethacrylamide) (NIPMAM)) microgels (TVPT = 45°C) [5] and poly(N-ethylmethacrylamide (NEMAM)) [6]. The elaboration of NEMAM based particles has been recently reported6 by investigating: (i) the effect of ionic initiator concentration on the polymerization of NEMAM in the presence of ethylene glycol dimethacrylate (EGDMA) as a crosslinker, (ii) the influence of EGDMA concentration on the polymerization mechanism and the final colloidal properties of the dispersions [7] and finally (iii) the functionalization process of NEMAM/EGDMA-based particles using phenyl boronic acid monomer [8]. Consequently, to prepare thermally sensitive particles having high TVPT, the crosslinker should be adequately selected with respect to its reactivity in polymerization. Poly(NEMAM)-based particles were obtained using ethylene glycol dimethacrylate (EGDMA) a hardly water-soluble crosslinker leading to monodisperse thermally-sensitive microgel particles exhibiting a high volume-phase-transition temperature TVPT, around 65 °C. The effect of EGDM concentration on polymerization kinetics, overall conversion, water-soluble polymer formation and final particle size was discussed on the basis of combination of an emulsion/precipitation polymerization processes and a polymerization mechanism was proposed [7].
As a part of this systematic work, we aim at reporting on the influence of the nature of crosslinker on polymerization kinetics, overall conversion and water-soluble polymer formation. In addition, particular attention is also paid to the effect of the chemical structure of crosslinker agent on the colloidal properties of the final particles such as hydrodynamic particle size distribution, electrokinetic study, volume-phase-transition temperature and swelling ability.
2 Experimental
2.1 Materials
Water was of milli-Q grade (Millipore SA, France) and was boiled for 2 h under a nitrogen stream before use. N-Ethylmethacrylamide (NEMAM) (from Polyscience, Warrington, PA, USA) was purified through alumina column. Potassium persulphate initiator (KPS) and sodium chloride were from Sigma (Saint-Quentin-Fallavier, France) and used as received. Ethylene glycol dimethacrylate (EGDMA) from Merck, 1,3-butanediol dimethacrylate (1,3-BDDMA) and 1,4-butanediol dimethacrylate (1,4-BDDMA) from Aldrich (Saint-Quentin-Fallavier, France) and tetraethylene glycol dimethacrylate (TEGDMA) from Fluka were used without further purification. Hydroquinone (from Jansen Chimica) was used as the inhibitor of the polymerization reaction.
2.2 Crosslinker solubility concentration in water
The solubility in water of the four crosslinkers used in this study was determined according to an analytical method described [9] elsewhere. A given crosslinker concentration was mixed with water under vigorous agitation. After phase separation, the amount of water-soluble crosslinker was then determined using UV analysis.
2.3 Preparation of P[NEMAM] particles
The polymerizations reactions were carried out under emulsifier-free conditions in a 50-ml thermostated round-bottomed four-necked flask, equipped with a glass anchor-shaped stirrer, condenser and nitrogen inlet. NEMAM and the crosslinker were first dissolved in boiled water and added in the polymerization reactor. The initiator was also dissolved in water and added when the polymerization temperature was equilibrated at 90 °C. The stirring rate was adjusted to 280 rpm. Samples were withdrawn in order to determine the polymerization conversion and particle size variations as a function of the polymerization time. Hydroquinone was used to stop the polymerization during the storage of the samples. The recipes of the prepared latexes are summarized as follows: 1 g of NEMAM, 0.01 g of KPS and 15% molar of a given crosslinker agent. The polymerizations were conducted during 6 h under nitrogen condition.
2.4 Methods
2.4.1 Conversion
The NEMAM monomer conversion as a function of time was determined using 1H-NMR technique (spectrometer Varian Unity Plus 500 MHz) by analysing the crude synthesized latex. The peaks ratio of the vinylic protons of the NEMAM monomer over the aliphatic O–CH2 protons of both the monomer and the copolymer led to conversion estimation. Then, the conversions were calculated from the initial and the determined residual NEMAM amount.
2.4.2 Water-soluble polymer (WSP)
The amount of formed water-soluble polymer was gravimetrically determined from the supernatant after centrifugation step. The supernatant was subsequently dried and the amount of water-soluble polymer was then deduced.
2.4.3 Particle size analysis
Particle size was measured by quasi-elastic light scattering (QELS) using the Zetasizer 3000HS from Malvern Instrument (Malvern, UK). The hydrodynamic diameter of a highly diluted latex dispersion in 10–3 M NaCl was measured as a function of polymerization time and temperature ranging from 20 to 70 °C. The samples were prepared using well boiled filtered and degassed Milli-Q water.
2.4.4 Size distribution
Transmission electron micrographs (TEM) were carried out using a Hitachi electron microscope (Hitachi S 800, CMEABG at Claude-Bernard University, Lyon-1, France). The particle size and size distribution were determined by measuring the size of a given number of particles. This technique was used to determine the particle size and size distribution of the microgel particles under dried state.
2.4.5 The volume phase-transition temperature
The volume phase-transition temperature (TVPT) was taken by measuring the optical density (OD) variation as function of temperature induced by the change in the dispersion turbidity attributed to the hydrodynamic particle size modification. The optical density was measured at 500 nm wavelength using a Uv=mc2 Spectrophotometer (Safas, Monaco). To cancel the effect of highly diluted latex particles concentration, the optical density variation as a function of temperature at a given condition was normalized.
2.4.6 Electrokinetic study
The electrophoretic mobility of microgel particles was measured as a function of pH in 10–3 M NaCl salt and at 20 °C and at a constant pH for various temperature using the Zetasizer 3000HS from Malvern Instrument (Malvern UK). According to the surface complexity, the measured electrophoretic mobilities cannot be easily converted to zeta potential as for the classical colloids.
3 Results and discussion
As already reported [7], a preliminary study has been investigated in order to examine the role of each reactant and to establish polymerization conditions leading to low water-soluble polymer formation and to narrowly size-distributed latex particles. Indeed, various recipes and polymerization conditions have been scanned using for instance MBA as a crosslinker agent (from 8 to 16 wt%), potassium persulphate as the initiator (0.6 to 4 wt%) and at 90 °C. Unsuccessfully, the size distributions reflected polydisperse particles and the conversions were ranging from 70 to 90% together with high water-soluble contents (from 30 to 60%). The obtained results have been attributed to the high MBA reactivity compared to that of NEMAM at the polymerization conditions (rapid MBA consumption). A means to reduce the reactivity of the cross-linker was to decrease its availability in the reaction mixture. Thus, various non-water-soluble crosslinkers were tested and special attention was paid to the effect of EGDM on the NEMA polymerization, as reported in a recent paper [8]. In addition, the effect of the initiator (potassium persulphate) on the copolymerization of NEMAM and EGDMA was reported by Hazot et al. [6]. Therefore in this work, the amount of crosslinker was maintained at a fixed concentration (i.e., 15%) in order to investigate the effect of the crosslinker nature on such combined emulsion/precipitation polymerization process.
3.1 Kinetic study
3.1.1 Conversion
The conversion of NEMAM plus the crosslinker was followed as a function of time and the obtained results are reported in Fig. 1 . Conversion versus time curves show that, polymerizations were almost complete within 150 min, irrespective of the crosslinker nature and that at conversions less than 60%, polymerization rates were roughly the same for all the investigated crosslinkers. The final conversion seemed to be slightly dependent upon the nature of the crosslinker (80% for TEGDMA and around 90% for the others) and this behaviour was principally attributed to the difference in their solubility in water (see Chart 1 ).
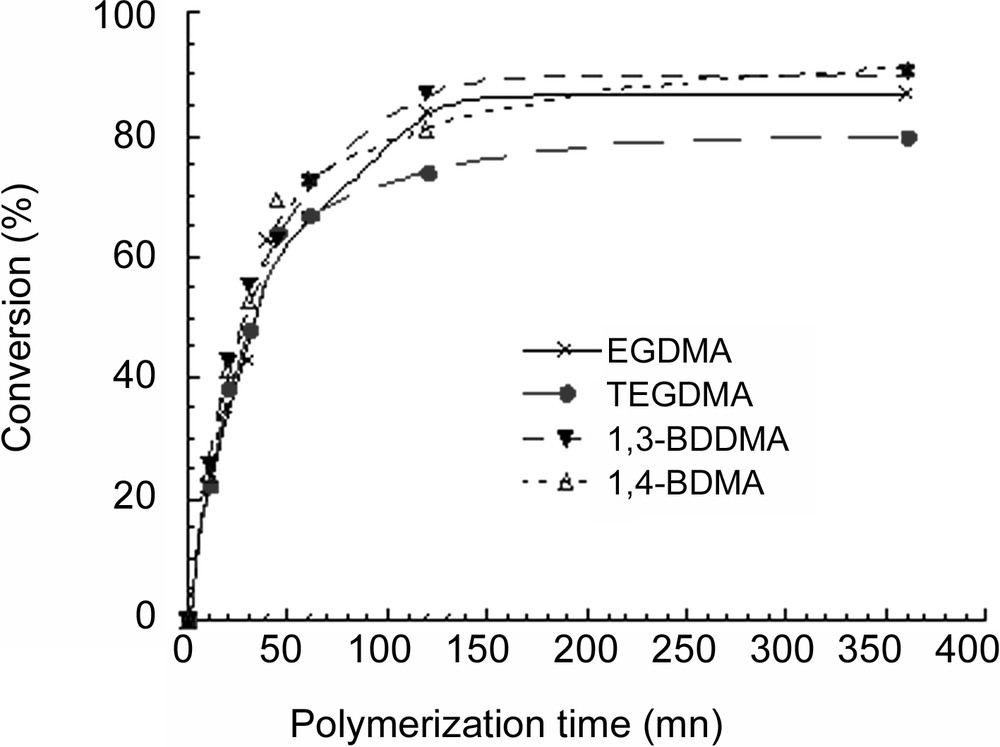
Conversion versus time of NEMAM for EGDMA (×), TEGDMA (●), 1,3-BDDMA (▾) and for 1,4-BDDMA (Δ).
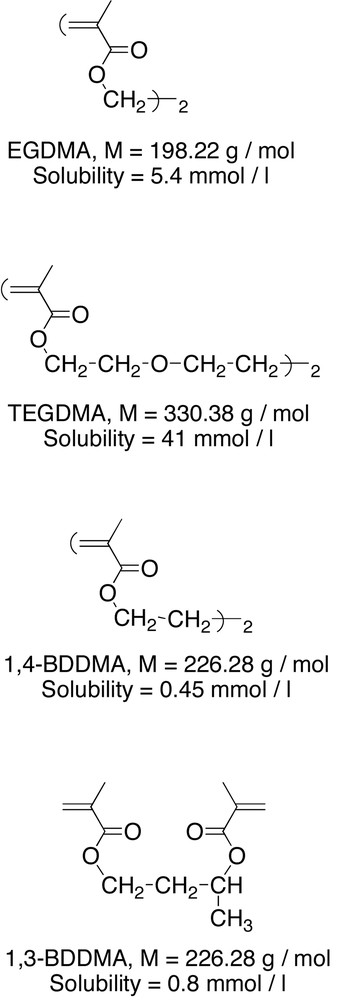
Chemical structure and solubility of EDGM, TEGDM, 1,4-BDDMA and 1,3-BDDMA.
TEGDMA, the more water-soluble crosslinker led to a large amount of water-soluble polymer and EGDMA, the least soluble, to the lowest. But water solubility is not the only parameter to be taken into account, as poorly soluble 1,3- and 1,4-BDDMA led to fair amounts of water soluble species. Hence, neither tangible explanation nor relationship can be directly established between the water-solubility of the crosslinker and the recovered amount of water-soluble polymer. This means that other parameters should be taken into consideration, such as for instance the reactivity of the crosslinker as well as its diffusion rate from the dispersed droplets to growing particles through the aqueous phase. Regarding reactivity, a rapid examination of their chemical structure does not allow us to clearly anticipate significant reactivity differences; however, the influence of steric hindrance could be invoked, mostly on the termination rate constant. Concerning diffusional aspects of the crosslinker along with the reaction and the obvious consequences on its partitioning, a relevant discussion is out of the scope of this work, since no rational investigation was performed in this direction. Nevertheless, the production of highly crosslinked poly(NEMAM) particles seems to be produced when TEGDMA is used as a consequence of its significant water solubility and probably higher reactivity in polymerization.
3.1.2 Particle size analysis
The particle size variation versus polymerization time was investigated for every crosslinker. In each case, the particle size reached a maximum within 100 min, as shown in Fig. 2 . Such a result may be explained by the high propagation rate of the polymerization during the first step of the reaction. As regard to the final particle size, the behaviour observed may be attributed to the difference in the water solubility concentration and the polymerization reactivity of the crosslinker agent and to water-soluble polymer formation. In fact, those parameters are directly related to the particles yield and consequently to the final particle size. The crude final latexes were also examined using TEM microscopy and the obtained micrographs are given in Fig. 3 . All latexes appear quite monodisperse in their dried state, which suggests the rapid formation of latex particles during the nucleation period. The diameters measured by TEM will not be discussed here since the particle sizes are measured under dried conditions, which are the combination of the shrunken state (induced by the dehydration) and the possible collapse of the microgel. However, it is interesting to observe that TEGDMA-crosslinked particles appear darker than the others, which could suggest a stronger crosslinker density. Such a behaviour was corroborated by an AFM investigation [7] showing that the aspect ratio (which expresses the degree of collapse of microgel particles) was the largest with the EGDMA-crosslinked particles and the lowest with the TEGDMA ones.
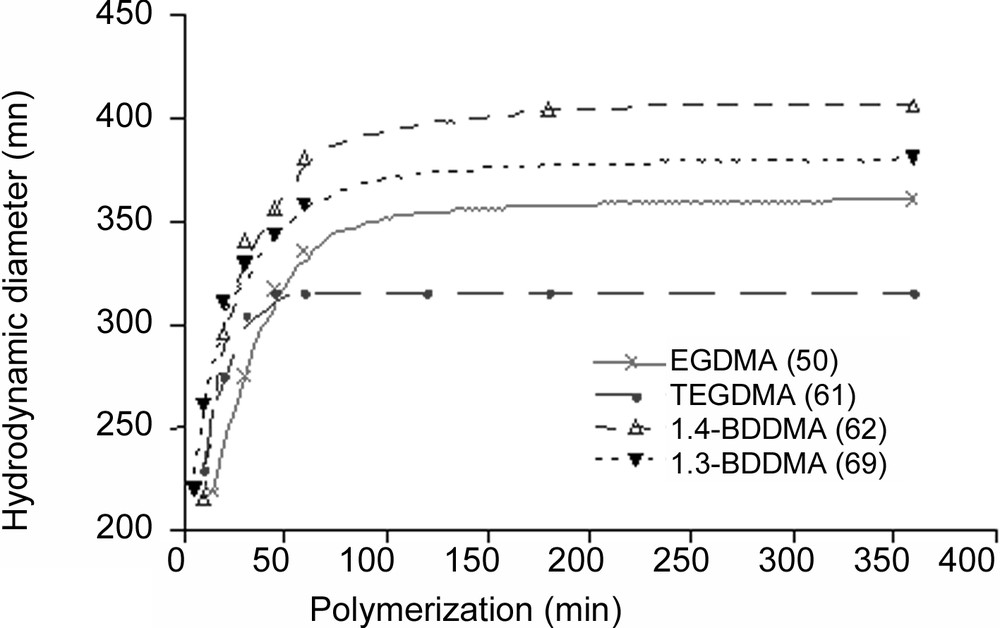
Effect of the crosslinker nature on hydrodynamic particle size measured at 20 °C as a function of time.
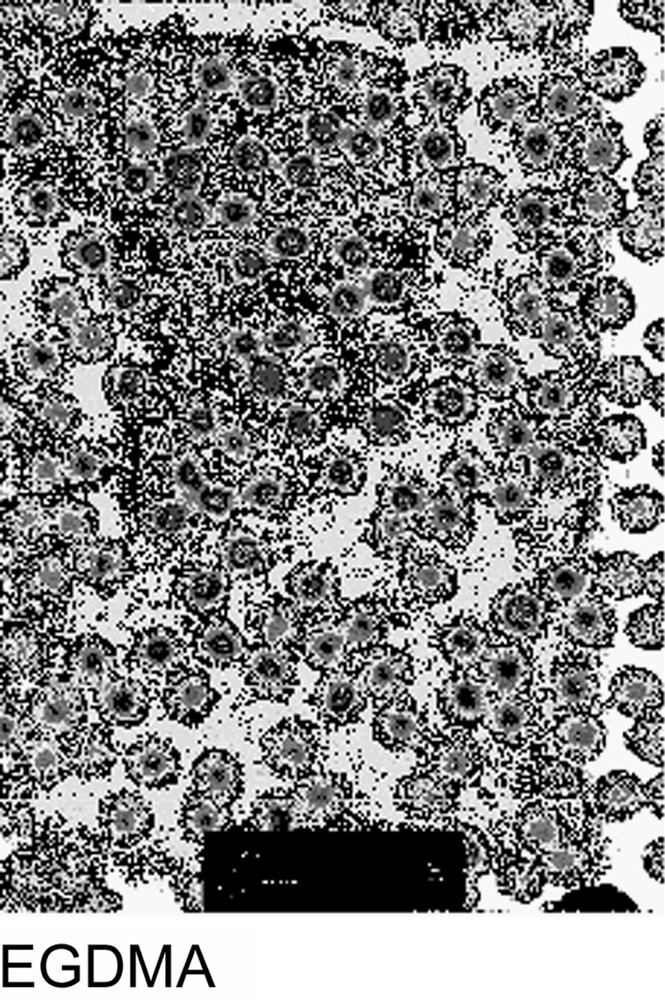

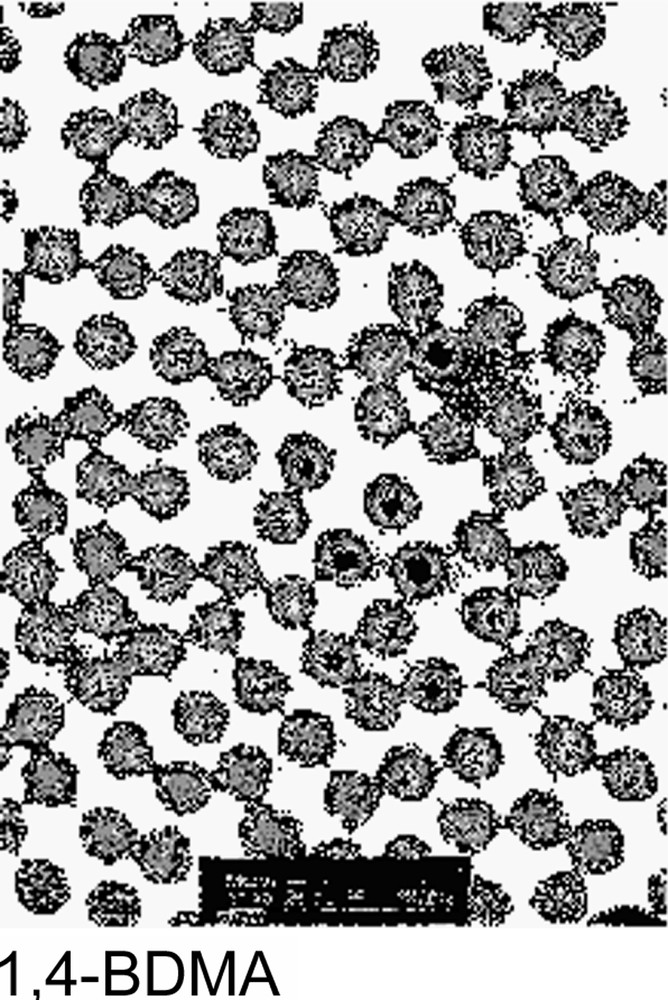
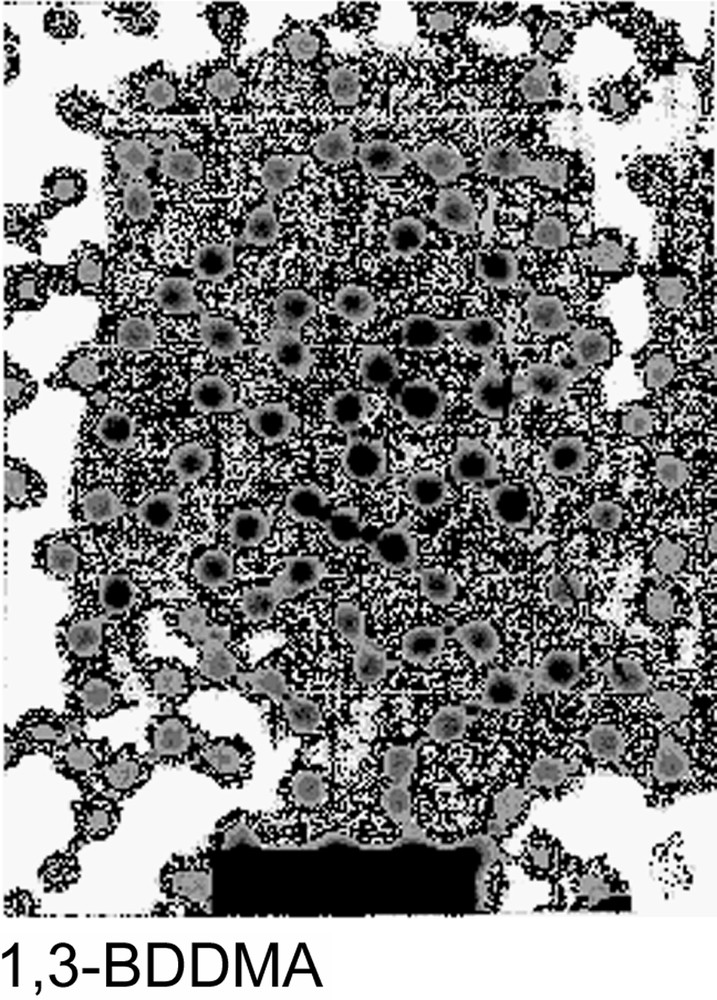
Transmission electron microscope of crosslinked poly(NEMAM) microsphere particles.
3.2 Colloidal characterization
3.2.1 volume-phase-transition temperature
The volume-phase-transition temperature (TVPT) of the prepared microgel particles was investigated using two classical methods: (i) the hydrodynamic particle size variation versus temperature and (ii) the turbidity variation of a highly diluted colloidal dispersion as a function of temperature.
The TVPT of the poly(NEMAM) particles was first obtained by measuring the hydrodynamic particle size (in 0.001 M NaCl) as a function of temperature ranging from 20 to 75 °C. As illustrated in Fig. 4 , the particle size decreased with increasing temperature, revealing the thermally-sensitive character of the obtained crosslinked microgel particles. This behaviour is characteristic of the shrinkage of the particles induced by the dehydration of poly(NEMAM) sequences. The estimated TVPT for each latex sample cannot be precisely deduced from such variation by considering the inflexion temperature point as the TVPT and only the transition temperature range can be deduced. In fact, the transition behaviour was found to occur over a broad temperature range centred in between 50 to 60 °C, and no drastic effect of the crosslinker agent nature is observed or clearly evidenced.
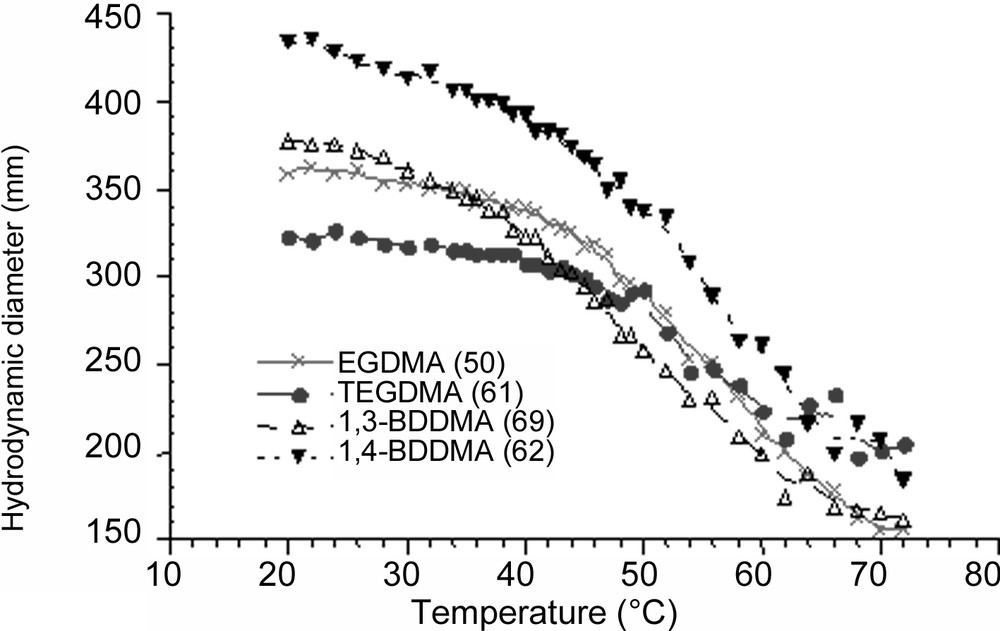
Particle size variation as a function of temperature at 0.001 M NaCl solution.
The difference in the particle size at 20 °C and above 60 °C provides a rough estimation of the swelling ability of the crosslinked microgel particles. The measured swelling ratio (SR = Dh/D70 °C)3 of such poly(NEMAM) based particles are found in between 12 and 3, the lowest value being observed when TEGDMA (the more water-soluble crosslinker) was used, giving the lowest polymer conversion (80%) and a large amount of WSP (68%). Due to the possible flattening of such soft particles, the swelling ability (Dh/DTEM)3 cannot be discussed using particle size measured by TEM.
The effect of temperature (T) on the optical density (OD) variation was examined and the OD measured versus T was normalized in order to cancel the effect of particles concentration and the curves (i.e. for various samples) obtained are reported in Fig. 5 . As can be observed from the OD versus T curves, the transition temperature takes place in a much broader temperature range than for uncrosslinked poly(N-alkylacrylamide) chains as well as for crosslinked poly(NIPMAM) [10] microspheres using MBA as a crosslinker agent. The noticed behaviour reflects that the more crosslinked the particles, the broader the temperature transition range. Such behaviour can be explained by a concentration gradient of the crosslinker from the core to the shell of the particle. Similar behaviour had been already reported by Duracher et al. [10]. In addition, it can be concluded (from Fig. 5) that there is no significant effect of the crosslinker nature on the TVPT, as already pointed out by hydrodynamic particle size analysis versus temperature (Fig. 4).
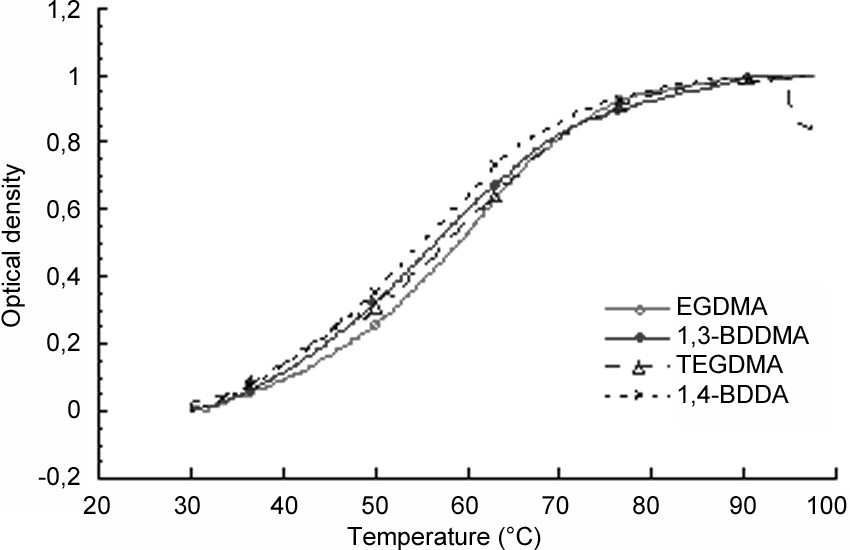
Optical density variation as a function of temperature of highly diluted latex dispersion in 0.001 M NaCl.
3.2.2 Electrokinetic study
Fig. 6 shows the electrophoretic mobilities of the prepared microgel particles as a function of pH, in 10–3 M NaCl solution at 20 °C. Particles exhibited a negative electrophoretic mobility above pH 3 for all latexes. The electrophoretic mobilities of latexes prepared using TEGDMA and 1,4-BDDMA decreased with increasing pH, whereas for 1-3 BDDMA and EGDMA latexes the decrease was quite limited. The observed negative electrophoretic mobility for all latexes was due to the sulphate groups originating from the decomposition of the persulphate initiator (KPS) with probably also the presence of carboxyl groups stemming from the hydrolysis of the sulphate groups [11]. The difference in the electrophoretic mobility profiles can reflect differences in the particle morphology according the type of crosslinker: (i) the heterogeneous charge distribution in the vicinity of the particle surface, (ii) the difference in the real shell thickness of the particles and (iii) the location of the charges contributing to the electrophoretic mobility. In fact, the plateau in the electrophoretic mobility measured at basic pH decreased as the swelling ratio decreases (see Table 1). The observed behaviour can be attributed to the sulphate charge dilution (originated from KPS) in the interfacial poly(NEMAM) shell. Then, the more crosslinked the shell, the higher the electrophoretic mobility at basic pH, which is indeed the case of particles, produced in the presence of TEGDMA.
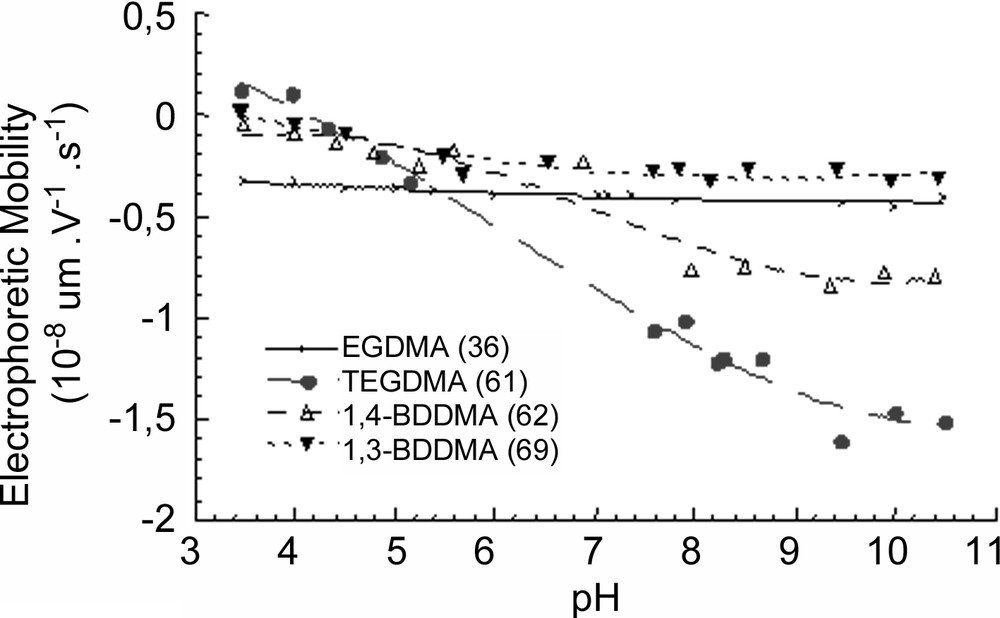
Electrophoretic mobility of latexes as a function of pH at a constant NaCl concentration 10–3 M and at 20 °C.
Solubility of the crosslinkers, polymerization conversion, water-soluble polymer formation, hydrodynamic particle size and swelling ability of the final latexes
| Solubility (mmol l–1) | Conversion(a) (wt%) | WSP(a) (wt.%) | Dh(b) (nm) | Dh(c) (nm) | SR(d) | |
| TEGDMA | 41 | 80 | 68 | 320 | 200 | 4.1 |
| EGDMA | 5.4 | 87 | 27 | 355 | 160 | 10.9 |
| 1,3-BDDMA | 0.8 | 90 | 72 | 370 | 160 | 12.3 |
| 1,4-BDDMA | 0.4 | 91 | 40 | 430 | 200 | 9.9 |
(a) The overall conversion (wt%) and water-soluble polymer amount (wt%) are gravimetrically and 1H-NMR determined, respectively.
(b) Hydrodynamic diameter determined using QELS at 20 °C and in 10–3 M NaCl.
(c) Hydrodynamic diameter determined using QELS at 70 °C and in 10–3 M NaCl.
In addition, such crosslinked particles exhibited high colloidal stability against ionic strength (the critical coagulation concentration, CCC, was above 1 molNaCl l–1, regardless of the crosslinker, results not shown), a behaviour confirming that SO4– terminated hydrophilic poly[NEMAM] chains imparted electrosteric stability.
4 Conclusion
Monodisperse and thermally-sensitive poly(NEMAM) latexes were prepared by batch polymerization process using various crosslinkers (EGDMA, 1-3BDDMA, 1-4BDDMA and TEGDMA). It was found that the nature of the crosslinker played a negligible role on the polymerization kinetics and overall conversions (particles plus water-soluble polymer) determined to be 80% by NMR. On the contrary, the water-soluble polymer amount was strongly dependent upon the nature of the crosslinker. The observed behaviour could not only be explained by considering the differences in the water-solubility of the crosslinker, other parameters such as the reactivity and diffusional aspects probably have to be taken into consideration.
Concerning the colloidal characterization of the final particles, the crosslinker was found to have a marginal influence on the volume-phase-transition temperature, revealing the rich poly(NEMAM) shell of the particles. Conversely, it was shown that the nature of the crosslinker dramatically affected the final hydrodynamic particle size and the electrophoretic mobility behaviour. Such a study proved to be useful for selecting EGDMA as a suitable crosslinker that indeed relatively fulfils the above mentioned requirements with regards to particle size distribution and low water-soluble polymer formation. It was then included in the various recipes later worked out, especially to prepare functionalized poly[NEMAM]particles.



Vous devez vous connecter pour continuer.
S'authentifier