1 Introduction
Knowledge of chemical compounds biodegradability is one of the most important aspects of their environmental behaviour. A biodegradable substance is expected to cause less ecological problems in the long term than a persistent one [1]. Polymer biodegradation test data are necessary before their marketing in order to know in which conditions they must be used. Degradation processes are constantly taking place on a large scale in the natural environment. The decomposers or ‘biodegraders’ are microorganisms, especially bacteria, and the decomposers implement several biochemical degradation pathways depending on natural substrates. In a laboratory, this natural degradation process has been simulated and facilitated by using liquid inorganic media in aerobic biodegradation tests. In this experimental set-up, biodegradation processes occurred under controlled conditions. Ultimate aerobic biodegradation consists in the breakdown of organic compounds by microorganisms into carbon dioxide, water and an increase of microbial biomass in the presence of oxygen.
The Organisation for Economic Cooperation and Development (OECD) is leading international efforts to standardize biodegradation test methods. The well-established OECD carbon dioxide (CO2) method [2] based on Sturm’s original test [3] is usually used for assessing biodegradability of more or less soluble organic chemicals. Evolution of carbon dioxide is considered by the OECD standard to be the only unequivocal proof of microbial activity [4]. The microbial inoculum may be extracted from a variety of sources: activated sludge or soils [5]. The aim of this work was to perfect a reliable method for testing the biodegradability of polymeric films under laboratory conditions.
The biodegradation test described here made allowance for recommendations of standards [2–9] and opted for a continuous-aerated system in which CO2 released into the headspace of bioreactors was measured with an infrared CO2 analyser. At defined regular intervals of time, a computer recorded instantaneous concentration of CO2 existing between air intake and air exit (differential method) in bioreactors. Automated measurement of CO2 is an advantage over the manual Sturm test [3]. This modified Sturm test procedure has been adapted to proteic mulching films using soil-extract microorganisms.
2 Experimental
2.1 Conditions of aerobic biodegradation test
All experiment vessels and mineral media [9] were sterilised by autoclaving at 121 °C and a pressure of 1 bar for 20 min. The water used in all the experiments was purified by filtration (MilliQ system®, Millipore, USA). All the reagents used were analytical grade.
The conditions of the biodegradation were based on standards for aerobic batch tests [1]. Bioreactors contained the tested film, the inorganic medium and soil microorganisms. So, the organic tested film was the only source of carbon and energy for the microorganisms. The standardized inorganic medium [9] had sufficient buffering capacity to maintain a pH value of about 7 throughout the biodegradation trial. According to standardized methods [5,9], the reaction medium initially contained about 106 colony-forming units (CFU) per millilitre. Test mixture thermostated at 23 °C was continuously aerated and mixed by stirring. Blank control bioreactor contained only inorganic medium [9] and microbial inoculum. For each trial, a set of six bioreactors (Fig. 1) was prepared as follows: (i) four bioreactors for the tested material, (ii) two blank bioreactors. Tested material and microbial inoculum were added into each bioreactor when CO2 saturation of liquid inorganic medium was reached.
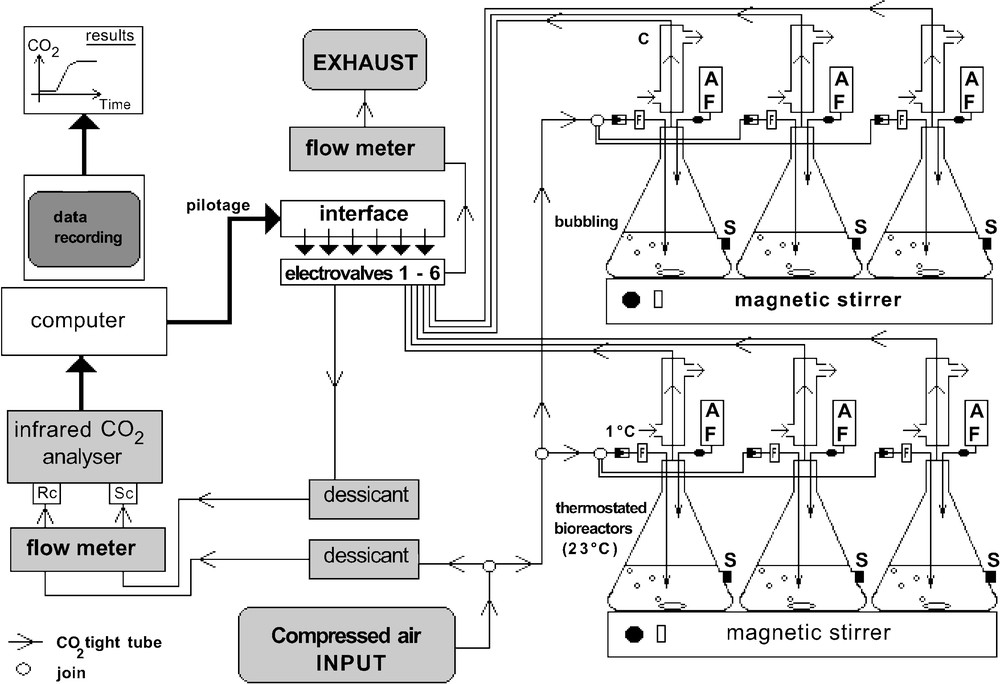
Scheme of automated experimental set-up for measuring polymer biodegradation (RC: reference cell, SC: sample cell, AF: addition funnel, F: sterile filter, S: septum, C: condenser).
The biodegradation test normally runs for 28 days [5], but may be prolonged or stopped depending on the occurrence of the plateau phase. The CO2 produced was measured in the exhaust air of each bioreactor at regular time intervals using a differential infrared CO2 analyser (IRGA).
The microbial source was obtained from samples of soil (30 g) aseptically collected in a sterile vessel from the upper layer (0–20 cm) of a field planted with vegetables located at Parence (Sarthe, France). Soil analysis gave 1.39% (percentage in dry mass) of organic carbon less than 4% as recommended by standards [10,11] (Microanalyses ICSN–CNRS, Gif-sur-Yvette, France). Soil pH was determined in a supernatant of slurry (5 parts distilled water, 1 part soil) [12,13]. The soil water pH was between 7 and 8, in accordance with standards [11].
2.2 Description of the experimental biodegradation set-up
This set-up is shown in Fig. 1. The analytical system is composed of a differential infrared gas (CO2) analyser (IRGA LI-6252, LI-COR®, Nebraska, USA). The analysis was based on the difference in absorption of infrared radiation passing through a reference cell (Rc) and a sampling cell (Sc). Tests were carried out using 3-l cylindrical thermoregulated (23 °C) bioreactors. All bioreactors were equipped with a check valve preventing reverse flow (VWR International SAS, Strasbourg-Cronenbourg, France), sterile filter (Ø = 0.22 μm, Nalgene®, USA) and magnetic stirring bar. Compressed air is flowed permanently into CO2-tight tubes [8] connecting the six bioreactors. Before analysis by IRGA, the air was regulated by flow meters (0.5 l min–1), dried through silica gel columns and sterilised by filtration. Airflow and stirring speed were adjusted at the beginning of the test. At regular intervals, the gaseous flow of each bioreactor was swept into the IRGA. The carbon dioxide given off is measured automatically and stored on a computer file. Biodegradation tests were monitored automatically by a homemade operating program which controlled opening and closing of each three-way electrovalve located downstream of each bioreactor. After the set period of analysis for one bioreactor, the computer switches on the electrovalves for another bioreactor. Moreover, during a biodegradation test, instantaneous concentration of CO2 and test parameters were displayed on the computer screen for the bioreactor in process. The level of biodegradation is calculated by comparing the CO2 given off with the theoretical amount (ThCO2) and expressed in a percentage.
2.3 Calculations
The theoretical amount of CO2 (ThCO2, g) produced by total oxidation of the tested material was calculated using the following equation:
| (1) |
From the kinetic of the formation of CO2 determined for each reactor, the cumulative amounts of carbon dioxide released were calculated in relation with time. The percentage of biodegradation (Pb) of the tested material for each measurement interval was expressed by:
| (2) |
2.4 Example of application to proteic film biodegradation
The proteic film was prepared with sunflower (Helianthus annuus L.) oilcakes plasticised with glycerol (20%, w/w) (V. Monti, personal communication). From the measured data, percentage of biodegradation is calculated and a biodegradation curve is drawn. The test result is obtained by comparing the measured CO2 with a theoretical value (ThCO2), which is calculated from the molecular formula of the reference substance and from elementary analysis (ponderal percentages) of the tested polymeric film according to calculations of standard ISO 14852 [9].
Fig. 2 shows the biodegradation degree of two proteic film samples with two different amounts as a function of time. This curve is distinguished by a lag phase (four days), a short exponential phase (four days) and a nearly linear phase. The start of the plateau phase only began after 27 days. Previous tests using the same proteic film (data not shown) but with microorganisms extracted from activated sludges showed that the plateau of the mineralisation curve versus time was reached sooner than with soil microorganisms. The percentage of the biodegradation here reached only 25%. This low level of mineralisation may be explained by the fact that a part of available carbon is used by microorganisms for heterotrophic growth. This large biomass production was verified at the end of each biodegradation test by cell counting.
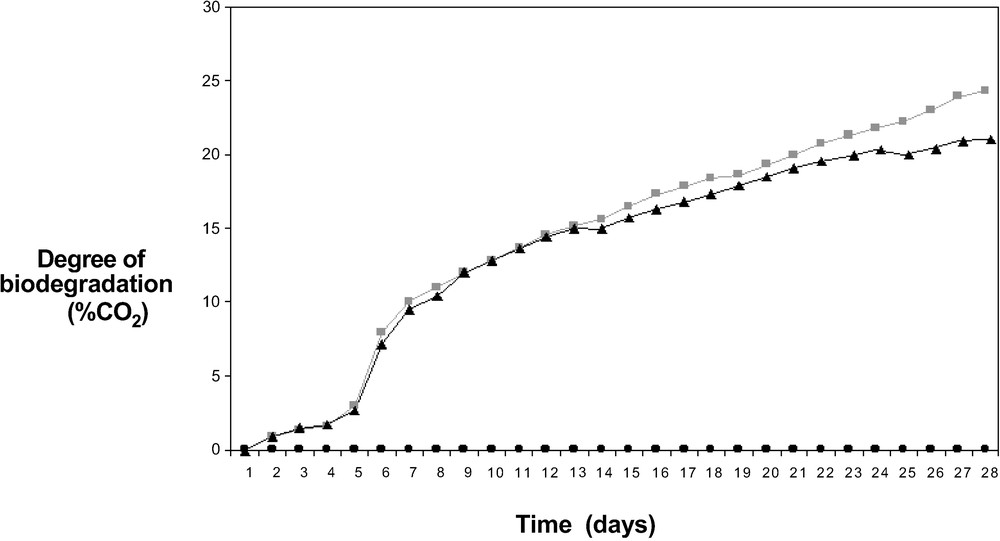
An example of biodegradation curves (mineralisation) as a function of time for two quantities of proteic film (full circle: blank (only soil microorganisms), full triangle: 0.5 g of initial proteic film, full square: 1 g of initial proteic film).
Whatever the pass level criteria are, good biodegradation [5,14] of a homogeneous test material is 60% in the case of tests using released CO2 as an indirect measure of biodegradation. Moreover, aqueous biodegradation tests have a considerably lower degradation potential compared to terrestrial tests, because fungi, which play an important part in polymer degradation, do not have optimum growth conditions in liquid medium.
3 Final remarks
For testing biodegradability on an appropriate laboratory-scale, this test method was available. The proteic film biodegradation by soil microorganisms was effective according to a molecular structure (spectrometric infrared analysis, data not shown). Therefore, in the case of new macromolecules, this aquatic test provides in the first step, basic information on potential biodegradation of such polymeric film. Moreover, biomolecular approach showed that the microbial community of soil extracts changed between the beginning and the end of the biodegradation test (data not shown). Consequently, there is a need for a clear identification of biodegradation actors that can be obtained with further biomolecular characterisation in order to normalise laboratory tests. Results of biodegradation tests are an important criterion for such material as polymeric mulching film, because the new European law [15] will classify them as environmentally hazardous or not.
Acknowledgements
The authors are grateful to ADEME and the ‘Région des Pays de la Loire’ for their support. Thanks are also extended to G. César, V. Monti, Y. Hardivillier, and N. Toquet for their precious help.


