1 Introduction
Although simple alkenes are not generally thought of as sites of nucleophilic attack, the formation of ring systems by the anionic cyclisation of olefinic alkyl, aryl and vinyllithiums is an interesting synthetic transformation and provides a regiospecific and highly stereoselective route to five-membered carbocycles [1,2] and heterocycles [3]. Most importantly, it should be possible to functionalize the initially formed cyclisation product by reaction with electrophiles, a reaction that is not generally possible in the case of radical cyclisations. A major drawback of this kind of carbocyclisations is that they are limited to terminal double bonds; however, it has been possible to obtain cyclised products for 1,2-disubstituted olefins in which the initially formed alkyllithium product is substituted with a moderately activating group [4] or with a leaving group in a β-position [5]. Although the development of this methodology for the preparation of heterocyclic systems has received less attention, several oxygen and nitrogen heterocycles, such as tetrahydrofurans [5,6], pyrrolidines [7], indolines [8,9] or indoles [10], have been synthesized via intramolecular carbolithiation reactions. Moreover, the fact that the ring closure of achiral olefinic organolithiums could proceed enantioselectively in the presence of (–)-sparteine dramatically increases the potential of this kind of processes [11,12]. In this area, we have studied in the last years the behaviour of 2-lithioallyl and 2-lithioaryl amines [13,14], as well as of 2-lithioaryl ethers [15], in their anionic cyclisations onto unactivated double bonds. Here we report our studies about the effect of different substituents at the terminal position of the allyl moiety in the intramolecular carbolithiation reaction of N-allyl-N-2-lithioallylamine derivatives.
2 Results and discussion
Sometime ago, we reported that N-allyl-N-(2-lithioallyl)amines 2, generated by bromine-lithium exchange from the corresponding N-allyl-N-(2-bromoallyl)amines 1, undergo a 5-exo intramolecular carbolithiation in the presence of N,N,N´,N´-tetramethylethylenediamine (TMEDA) to afford 3-lithiomethyl-4-methylenepyrrolidines 3 when the starting amine is aliphatic (R = alkyl) [13]. However, if the 2-bromoallylamine 1 is aromatic (R = aryl), the secondary amide 4 is generated (Fig. 1). In this case, we initially proposed a 6-endo cyclisation followed by an irreversible β-elimination (for an alternative mechanism involving an exo cyclisation of organolithium 3, γ-elimination, and a rapid and irreversible fragmentation of the corresponding cyclopropyl intermediate, see [3]). Aside from the mechanistic pathways, these cyclisations provide an efficient method for the preparation of methylenepyrrolidine derivatives.

Intramolecular carbolithiation of N-allyl-N-(2-lithioallyl)amines 2.
In order to investigate the effect of the substituents of the allyl moiety of the starting amine in the outcome of the intramolecular cyclisation, we decided to prepare several allyl amines substituted at the terminal position. The syntheses of these compounds 6 were carried out according to Fig. 2. The commercially available N-allyl-N-cyclohexylamine or N-allylaniline was selectively functionalized by successive treatment with n-BuLi and t-BuLi and further reaction with electrophiles (chlorotrimethylsilane, diphenyldisulfide or tributyltin chloride), affording secondary amines 5a-d as the Z-isomers [16]. On the other hand, the aromatic amines 5e–f were easily obtained by alkylation of aniline with cinnamyl and crotyl bromide, respectively. Finally, tertiary amines 6 were prepared by alkylation of the corresponding amine 5 with 2,3-dibromopropene using potassium carbonate as base (Table 1).

Synthesis of N-(3-substituted-2-propenyl)-N-(2-bromoallyl)amines 6.
Preparation of tertiary N-(2-bromoallyl)amines 6
| Starting amine | R | G | Product | Yield (%)a |
| 5a | c-C6H11 | (Z)-SPh | 6a | 72 |
| 5b | c-C6H11 | (Z)-SiMe3 | 6b | 71 |
| 5c | c-C6H11 | (Z)-SnBu3 | 6c | 79 |
| 5d | Ph | (Z)-SiMe3 | 6d | 83 |
| 5e | Ph | (E)-Ph | 6e | 81 |
| 5f | Ph | Meb | 6f | 75 |
a Isolated yields based on starting amines 5.
b Mixture of E:Z-diastereoisomers (6:1).
Treatment of N-(2-bromoallyl) amines 6 with 2 equiv. of t-BuLi at –78 °C in diethyl ether afforded the vinyllithium derivatives 7. Addition of TMEDA (2.2 equiv) at low temperature, warming up of the mixture until 0 °C and further reaction with different electrophiles allowed the isolation, after hydrolysis and purification by column chromatography, of 3-substituted-4-methylenepyrrolidines 8 in good yields (Fig. 3 and Table 2). The formation of these pyrrolidine derivatives can be understood by assuming a 5-exo intramolecular carbolithiation process that would give rise to the organolithium intermediates 9, which are finally functionalized by treatment with electrophiles.

Intramolecular carbolithiation of N-(2-lithioallyl)amines 7.
Synthesis of 3-functionalized-4-methylenepyrrolidines 8
| Starting amine | Organolithium compound | R | G | E | Product | Yield (%)a |
| 6a | 7a | c-C6H11 | SPh | D | 8a | 92 |
| 6a | 7a | c-C6H11 | SPh | SPh | 8b | 90 |
| 6a | 7a | c-C6H11 | SPh | SnBu3 | 8c | 83b |
| 6a | 7a | c-C6H11 | SPh | C(OH)Ph2 | 8d | 84b |
| 6b | 7b | c-C6H11 | SiMe3 | D | 8e | 90 |
| 6b | 7b | c-C6H11 | SiMe3 | SiMe3 | 8f | 80 |
| 6b | 7b | c-C6H11 | SiMe3 | SPh | 8g | 85b |
| 6b | 7b | c-C6H11 | SiMe3 | SnBu3 | 8h | 73b |
| 6c | 7c | c-C6H11 | SnBu3 | D | 8i | 94 |
| 6d | 7d | Ph | SiMe3 | D | 8j | 53c |
| 6d | 7d | Ph | SiMe3 | SPh | 8k | 50b,c |
| 6d | 7d | Ph | SiMe3 | SnBu3 | 8l | 55b,c |
| 6e | 7e | Ph | Ph | D | 8m | 61 |
a Isolated yields based on starting amines 6.
b Yields referred to the mixture of diastereoisomers.
c Low yields obtained due to a partial decomposition of the product on the purification by silica gel chromatography.
In those cases in which the E and G groups are different, pyrrolidine derivatives 8 were obtained as an approximately 2:1 mixture of diastereoisomers. This fact seems to indicate that if we assume that carbolithiation reactions are stereospecific syn-addition processes, organolithiums 9 must be configurationally labile at the temperature required to achieve the cyclisation [17,18].
The lack of formation of pyrrolidine derivatives starting from amine 6f, with a methyl group at the terminal position of the double bond, could be attributed to the fact that in the carbolithiation step a non-stabilized secondary carbanion would be generated.
It is interesting to note the effect that the trimethylsilyl and phenyl groups at the terminal position of the allyl moiety exert on the regioselectivity of the process when the starting amine is aromatic (R = Ph). Organolithium compounds 7d and 7e undergo regioselectively 5-exo cyclisations giving rise to pyrrolidine derivatives 8j-m. These results contrast with our previous report where aromatic N-allyl-N-(2-lithioallyl)amines undergo a 6-endo cyclisation processes [13] (see Fig. 1). So, the presence of the trimethylsilyl or phenyl groups at the terminal position of the double bond not only favours the carbolithiation of 1,2-disubstituted alkenes but also directs it to a 5-exo closure. So, the observed regioselectivity of the cyclisation of aromatic amines is inverted when the double bond is substituted at the terminal position with a moderately activating group such as phenyl or trimethylsilyl.
Moreover, although carbolithiation reactions of phenyl, trimethylsilyl or phenylthio-substituted double bonds are known [4,19], in this paper we present the first example of this kind of carbocyclisations in which the alkene moiety to be carbolithiated is functionalized with a tributyltin group (see organolithium 7c). Surprisingly, this reaction takes place almost quantitatively though it is well known the easy tin-lithium transmetalation reaction.
In order to know the temperature at which the carbolithiation processes take place for each of the N-2-(lithioallyl)amines 7, the cyclisations were monitored by GC–MS analysis of aliquots quenched with MeOD at different temperature intervals. After this study, we can conclude that in all the cases the temperature at which the cyclisation reaction takes place is lower than the temperature at which the unsubstituted parent N-allyl-N-(2-lithioallyl)amines 2 cyclises (see Fig. 1). In addition, some interesting differences were found depending on the G group and on the nitrogen electron density. So, organolithium compound 7a, with a phenylthio substituent at the allyl moiety, cyclises at –78 °C, whereas 7b, substituted with a trimethylsilyl group, cyclises at –20 °C. On the other hand, the exact temperature at which the cyclisation of tributyltin-substituted 7c occurs could not be determined with accuracy. However, we could estimate that, in this, case the cyclisation step proceeds at temperature lower than –50 °C. In the case of the organolithium compound 7d, derived from an aromatic amine and activated with a trimethylsilyl group, the cyclisation takes place at –50 °C and so, by comparing with 7b, we can conclude that the carbolithiation reactions are faster with aromatic amines than with aliphatic ones. This result could be interpreted taking into account that aliphatic amines could coordinate better with the lithium atom in the organolithium compounds 7, decreasing the reactivity of these intermediates.
Taking into account the activation effect of a phenylthio group on the double bond and with the aim of extending this reactivity to other starting amines, N-(2-bromo-2-cyclohexenyl)-N-[(Z)-3-phenylthio-2-propenyl]cyclohexylamine 10 was synthesized by alkylation of secondary amine 5a with 1,6-dibromocyclohexene [20] in the usual way (Fig. 4). Treatment of 10 with t-BuLi at –78 ºC rendered organolithium derivative 11 by a bromine-lithium exchange. Addition of TMEDA (2.2 equiv) at the same temperature and warming to –60 °C followed by reaction with MeOH or Ph2S2 from –78 to 20 °C afforded hexahydroindole derivatives 12a,b in excellent yields and as single diastereoisomers. The structure of compounds 12 and the anti relationship of H1 and H2 were unequivocally ascertained by 2D-NMR experiments (COSY, HMQC, HMBC, and NOESY). Again, the formation of the bicyclic compounds 12 can be explained by assuming a 5-exo intramolecular carbolithiation process that gives rise to the organolithium intermediate 13. Further reaction with the corresponding electrophile produces the final products 12 (Fig. 4).
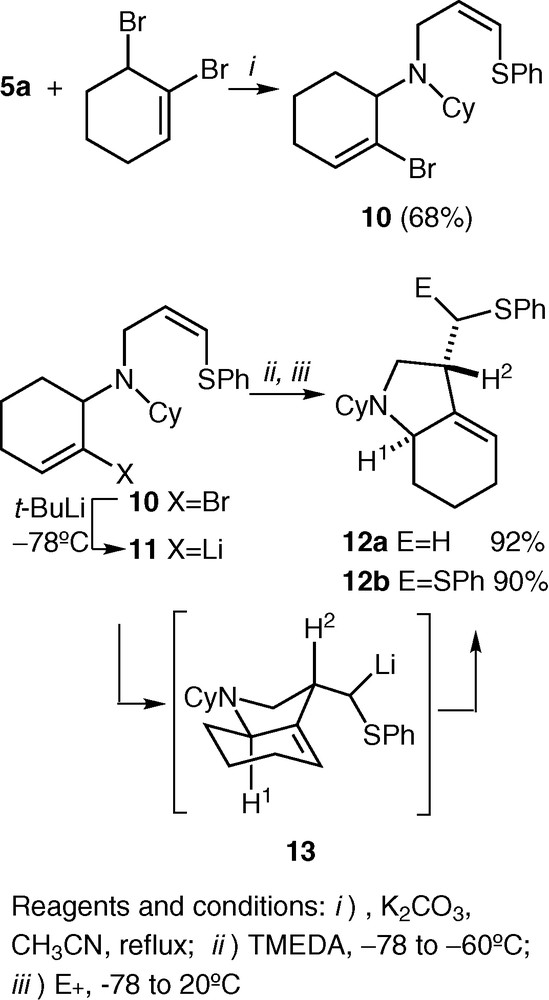
Diastereoselective intramolecular carbolithiation of organolithium compound 11.
The observed diastereoselectivity of this cyclisation is consistent with a four-centre transition state similar to the one proposed by Chamberlin for the synthesis of related methylenecyclopentane derivatives [21]. A preferred coplanar approach of the C–Li bond to the double bond would give the observed product (Fig. 5).

Proposed transition state for the cyclisation of organolithium 11.
In conclusion, we have described the intramolecular carbolithiation of N-allyl-N-2-lithioallylamines that present a moderately activating group at the terminal position of the double bond. These groups favour the cyclisation and the 5-exo regioselectivity. The first example of this kind of reactions with a tributyltin-substituted olefin has also been presented. Following this strategy, interesting functionalized pyrrolidine and hexahydroindole derivatives have been synthesized.
3 Experimental part
3.1 General remarks
Experiments involving organometallics were carried out in dried glassware under an atmosphere of dry nitrogen using standard Schlenk techniques. Liquid nitrogen was used as a cryoscopic fluid. All common reagents and solvents were obtained from commercial suppliers and used without further purification, unless otherwise indicated. n-BuLi was used as a 2.5-M solution in hexane. t-BuLi was used as a 1.5-M solution in pentane. THF and Et2O were freshly distilled from sodium-benzophenone ketyl prior to use. TLC was performed on Al-backed plates coated with silica gel 60 with F254 indicator (Merck). Flash column chromatography was carried out over Merck silica gel 60. 1H NMR and 13C NMR spectra were recorded on a Varian Inova 400 (400 and 100.6 MHz, respectively), Varian Gemini VXR-200 (200 and 50.3 MHz, respectively) and Bruker AC-300 (300 and 75.5 MHz, respectively). Chemical shifts are reported in δ relative to an internal standard of residual chloroform (δ = 7.27 for 1H NMR and δ = 76.95 for 13C NMR). Low-resolution electron impact mass spectra (EI–LRMS) were obtained at 70 eV on a HP 5971 A instrument, and the intensity of the molecular peak is reported as a percentage relative to the base peak after the corresponding m/z value. Elemental analyses were performed with a LECO CHNS-932.
3.2 General preparation of compounds 6 and 10
The corresponding secondary amine 5 (10 mmol), K2CO3 (10 mmol), 2,3-dibromopropene or 1,6-dibromocyclohexene (10 mmol), and acetonitrile (20 ml) were placed in a flask. The mixture was heated at reflux overnight. The solvent was removed (15 mm Hg), the residue was extracted with Et2O (3 × 10 ml), dried over Na2SO4 and the residue purified by column chromatography to afford amines 6 and 10.
3.2.1 N-(2-Bromoallyl)-N-[(Z)-3-phenylthioallyl]cyclohexylamine 6a
From amine 5a (2.64 g, 72%). Rf = 0.28 (hexane/AcOEt, 30:1). 1H NMR (400 MHz, CDCl3): δ 7.36–7.18 (m, 5H, ArH), 6.31–6.26 (m, 1H, CH=CHS), 6.01–5.99 (m, 1H, C(Br)=CHH), 5.97–5.85 (m, 1H, CH=CHS), 5.54–5.52 (m, 1H, C(Br)=CHH), 3.36 (dd, J = 6.4 and 1.2 Hz, 2H, NCH2CH=), 3.30 (s, 2H, NCH2C(Br)=), 2.60–2.49 (m, 1H, NCH), 1.90–1.10 (m, 10H, 5 × CH2 cyclohexyl). 13C NMR (50.3 MHz, CDCl3): δ 134.1, 131.4, 129.0, 128.9, 126.3, 124.6, 123.5, 116.7, 59.8, 58.6, 48.5, 29.2, 26.2, 26.1. EI-LRMS, m/z (%): 367 (5) [M++2], 365 (5) [M+], 149 (100). Elemental analysis calcd (%) for C18H24BrNS (366.4): C 59.01, H 6.60, N 3.82; found C 59.08, H 6.53, N 3.92.
3.2.2 N-(2-Bromoallyl)-N-[(Z)-3-trimethylsilylallyl]cyclohexylamine 6b
From amine 5b (2.34 g, 71%). Rf = 0.3 (hexane). 1H NMR (400 MHz, CDCl3): δ 6.32 (dt, J = 14.4 and 6.8 Hz, 1H, CH=CHSi), 5.98–5.96 (m, 1H, C(Br)=CHH), 5.58 (dt, J = 14.4 and 1.6 Hz, 1H, CH=CHSi), 5.51–5.49 (m, 1H, C(Br)=CHH), 3.25 (s, 2H, NCH2C(Br)=), 3.22 (dd, J = 6.8 and 1.6 Hz, 2H, NCH2CH=), 2.54–2.44 (m, 1H, NCH), 1.81–1.73 (m, 4H, cyclohexyl), 1.64–1.57 (m, 1H, cyclohexyl), 1.28–0.98 (m, 5H, cyclohexyl), 0.11 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 148.0, 134.2, 130.4, 116.4, 59.4, 58.1, 52.3, 29.3, 26.2, 26.0, 0.20. EI-LRMS, m/z (%): 331 (8) [M++2], 329 (8) [M+], 250 (100), 73 (98). Elemental analysis calcd (%) for C15H28BrNSi (330.4): C 54.53, H 8.54, N 4.24; found C 54.65, H 8.48, N 4.31.
3.2.3 N-(2-Bromoallyl)-N-[(Z)-3-tributyltinallyl]cyclohexylamine 6c
From amine 5c (4.32 g, 79%). Rf = 0.4 (hexane). 1H NMR (400 MHz, CDCl3): δ 6.51 (dt, J = 12.4 and 6.4 Hz, 1H, CH=CHSn), 5.99–5.97 (m, 1H, C(Br)=CHH), 5.95 (dt, J = 12.4 and 1.6 Hz, 1H, CH=CHSn), 5.51–5.49 (m, 1H, C(Br)=CHH), 3.25 (s, 2H, NCH2C(Br)=), 3.15 (dd, J = 6.4 and 1.6 Hz, 2H, NCH2CH=), 2.53–2.48 (m, 1H, NCH), 1.78–0.80 (m, 37H, 5 × CH2 cyclohexyl and 3 × (CH2)3CH3 SnBu3). 13C NMR (100.6 MHz, CDCl3): δ 148.1, 134.3, 129.8, 116.3, 59.4, 58.0, 56.0, 29.2, 29.1, 27.3, 26.3, 26.1, 13.7, 10.3. EI-LRMS, m/z (%): 490 (7) [M+-C4H9], 313 (29), 256 (30), 176 (100). Elemental analysis calcd (%) for C24H46BrNSn (547.2): C 52.67, H 8.47, N 2.56; found C 52.81, H 8.60, N 2.51.
3.2.4 N-(2-Bromoallyl)-N-[(Z)-3-trimethylsilylallyl]aniline 6d
From amine 5d (2.69 g, 83%). Rf = 0.38 (hexane). 1H NMR (400 MHz, CDCl3): δ 7.40–7.30 (m, 2H, ArH), 6.91–6.81 (m, 3H, ArH), 6.49 (dt, J = 14.6 and 6.2 Hz, 1H, CH=CHSi), 5.97–5.87 (m, 2H, CH=CHSi and C(Br)=CHH), 5.69–5.67 (m, 1H, C(Br)=CHH), 4.22–4.13 (m, 4H, 2 × NCH2), 0.34 (s, 9H, 3 × CH3 SiMe3). 13C NMR (50.3 MHz, CDCl3): δ 147.6, 144.6, 132.5, 129.6, 129.2, 117.3, 115.8, 112.2, 58.5, 52.0, 0.1. EI-LRMS, m/z (%): 325 (40) [M++2], 323 (40) [M+], 244 (52), 224 (64), 73 (100). Elemental analysis calcd (%) for C15H22BrNSi (324.3): C 55.55, H 6.84, N 4.32; found C 55.48, H 6.75, N 4.39.
3.2.5 N-(2-Bromoallyl)-N-[(E)-cinnamyl]aniline 6e
From amine 5e (2.66 g, 81%). Rf = 0.33 (hexane:AcOEt, 40:1). 1H NMR (300 MHz, CDCl3): δ 7.40–6.80 (m, 10H, ArH), 6.60 (d, J = 15.9 Hz, 1H, PhCH=), 6.35–6.25 (m, 1H, CH2CH=), 5.80 (t, J = 1.7 Hz, 1H, C(Br)=CHH), 5.60 (t, J = 1.7 Hz, 1H, C(Br)=CHH), 4.20–4.15 (m, 4H, 2 × NCH2). 13C NMR (75.5 MHz, CDCl3): δ 147.5, 136.5, 131.4, 129.4, 129.2, 128.4, 127.4, 126.2, 124.8, 117.2, 115.8, 112.1, 58.3, 52.2. Elemental analysis calcd (%) for C18H18BrN (328.2): C 65.86, H 5.53, N 4.27; found C 65.58, H 5.71, N 4.29.
3.2.6 N-(2-Bromoallyl)-N-(2-butenyl)aniline 6f
From amine 5f (1.99 g, 75%, mixture of Z,E-diastereoisomers). Rf = 0.43 (hexane:AcOEt, 25:1). Data from the (E) diastereoisomer: 1H NMR (300 MHz, CDCl3): δ 7.30–6.70 (m, 5H, ArH), 5.80–5.50 (m, 4H, CH=CH and C(Br)=CH2), 4.10 (s, 2H, NCH2C(Br)), 3.90 (d, J = 5.2 Hz, 2H, NCH2CH=), 1.70 (d, J = 6.0 Hz, 3H, CH3). 13C NMR (50.3 MHz, CDCl3): δ 147.6, 129.6, 129.0, 127.7, 125.8, 116.9, 115.6, 112.0, 58.2, 47.1, 17.6. EI-LRMS, m/z (%): 267 (43) [M++2], 265 (46) [M+], 186 (100). Elemental analysis calcd (%) for C13H16BrN (266.2): C 58.66, H 6.06, N 5.26; found C 58.50, H 6.01, N 5.09.
3.2.7 N-(2-Bromo-2-cyclohexenyl)-N-[(Z)-3-phenylthioallyl]aniline 10
From amine 5a (2.76 g, 68%). Rf = 0.26 (hexane). 1H NMR (400 MHz, CDCl3): δ 7.36–6.95 (m, 5H, ArH), 6.30–6.26 (m, 1H, C(Br)=CH), 6.21 (dt, J = 9.2 and 1.6 Hz, 1H, CH=CHS), 6.02–5.95 (m, 1H, CH=CHS), 3.59–3.49 (m, 2H, NCH2CH=), 3.31 (ddd, J = 15.6, 4.8 and 2.0 Hz, 1H, NCHC(Br)=), 2.63–2.54 (m, 1H, NCH), 2.14–1.00 (m, 16H, 5 x CH2 cyclohexyl and 3 × CH2 cyclohexenyl). 13C NMR (100.6 MHz, CDCl3): δ 136.4, 134.7, 133.2, 129.1, 128.9, 128.6, 126.1, 122.5, 59.5, 58.4, 44.2, 32.6, 31.7, 28.8, 27.6, 26.4, 26.3, 26.2, 20.9. EI-LRMS, m/z (%): 407 (2.3) [M++2], 405 (1.3) [M+], 149 (100). Elemental analysis calcd (%) for C21H28BrNS (406.4): C 62.06, H 6.94, N 3.45; found C 61.95, H 6.98, N 3.37.
3.3 General preparation of compounds 8 and 12
A solution of the corresponding 2-bromoallylamine 6 or 10 (2 mmol) in Et2O (15 ml) was treated with 2 equiv of t-BuLi (4 mmol, 2.67 ml of a 1.5 M solution in pentane) at –78 ºC. The mixture was stirred for 20 min at this temperature, and then TMEDA (4.4 mmol, 0.66 ml) was added. The resulting mixture was stirred for 2 h at –78 ºC in the case of amine 6a, for 2 h at –20 °C in the case of amine 6b, for 30 min at 0 °C in the case of amine 6c and for 1 h at –50 ºC in the case of aromatic amine 6d. In all the cases, the ethereal solution of the cyclised anions 9 and 13 was cooled to –78 °C and 1.1 equiv (2.2 mmol) of the corresponding electrophile (deuterium oxide, chlorotrimethylsilane, tributyltin chloride, diphenyl disulfide, benzophenone) was added. Then, the mixture was allowed to reach room temperature, and the reaction was further stirred for 3 h. The mixture was hydrolysed with water and extracted with Et2O (3 × 10 ml). The combined organic layers were dried over anhydrous Na2SO4 and the resulting residue was purified by column chromatography yielding compounds 8 and 12.
3.3.1 1-Cyclohexyl-3-(1-deuterio-1-phenylthiomethyl)-4-methylenepyrrolidine 8a
From amine 6a (0.53 g, 92%). Rf = 0.28 (hexane/AcOEt, 1:1). 1H NMR (400 MHz, CDCl3): δ 7.35–7.31 (m, 2H, ArH), 7.28–7.22 (m, 2H, ArH), 7.17–7.12 (m, 1H, ArH), 4.97–4.95 (m, 1H, =CHH), 4.93–4.90 (m, 1H, =CHH), 3.38 (d, J = 13.6 Hz, 1H, NCHHC=), 3.19 (d, J = 4.8 Hz, 1H, CHCHDS), 3.14 (ddd, J = 13.6, 4.4 and 2.0 Hz, 1H, NCHHC=), 3.04 (dd, J = 9.2 and 7.2 Hz, 1H, NCHHCH), 2.88–2.80 (m, 1H, CHCHDS), 2.43 (dd, J = 9.2 and 6.8 Hz, 1H, NCHHCH), 2.00–1.50 (m, 6H, cyclohexyl and NCH), 1.29–1.11 (m, 5H, cyclohexyl). 13C NMR (100.6 MHz, CDCl3): δ 150.9, 136.3, 129.0, 128.7, 125.8, 105.5, 63.3, 57.5, 57.1, 41.6, 37.5 (t, J = 21.3 Hz), 31.4, 25.9, 24.8. EI-LRMS, m/z (%): 288 (1) [M+], 165 (100). Elemental analysis calcd (%) for C18H24DNS (288.5): C 74.94, H/D 9.08, N 4.86; found C 74.88, H/D 9.01, N 4.93.
3.3.2 3-[1,1Bis(phenylthio)methyl]-1-cyclohexyl-4-methylenepyrrolidine 8b
From amine 6a (0.71 g, 90%). Rf = 0.2 (hexane/AcOEt, 2:1). 1H NMR (400 MHz, CDCl3): δ 7.47–7.42 (m, 2H, ArH), 7.42–7.37 (m, 2H, ArH), 7.31–7.20 (m, 6H, ArH), 5.10–5.07 (m, 1H, =CHH), 5.07–5.04 (m, 1H, =CHH), 4.59 (d, J = 3.6 Hz, 1H, CHS2), 3.58 (d, J = 12.8 Hz, 1H, NCHHC=), 3.34–3.22 (m, 2H, NCHHCH), 3.10 (dd, J = 12.8 and 2.4 Hz, 1H, NCHHC=), 2.58 (t, J = 8.0 Hz, 1H, NCHHCH), 2.12–2.05 (m, 1H, NCH), 2.00–1.55 (m, 5H, cyclohexyl), 1.32–1.14 (m, 5H, cyclohexyl). 13C NMR (100.6 MHz, CDCl3): δ 148.3, 134.8, 134.6, 132.3, 132.2, 128.8, 128.7, 127.5, 127.4, 106.5, 63.0, 62.4, 58.1, 54.2, 46.2, 31.2, 31.1, 25.8, 24.6, 24.5. EI-LRMS, m/z (%): 395 (0.05) [M+], 164 (100). Elemental analysis calcd (%) for C24H29NS2 (395.6): C 72.86, H 7.39, N 3.54; found C 72.78, H 7.31, N 3.64.
3.3.3 1-Cyclohexyl-3-(1-phenylthio-1-tributyltinmethyl)-4-methylenepyrrolidine 8c
From amine 6a (0.96 g, 83%). Rf = 0.2 (hexane/AcOEt, 10:1). Data from major diastereoisomer: 1H NMR (400 MHz, CDCl3): δ 7.36–7.32 (m, 2H, ArH), 7.29–7.23 (m, 2H, ArH), 7.16–7.10 (m, 1H, ArH), 4.94–4.91 (m, 1H, =CHH), 4.88–4.84 (m, 1H, =CHH), 3.31–3.08 (m, 4H, NCHHC=, CHCHSSn), 2.92 (dd, J = 8.8 and 7.2 Hz, 1H, NCHHCH), 2.55 (dd, J = 8.8 and 5.6 Hz, 1H, NCHHCH), 2.00–1.42 (m, 12H, NCH, SnBu3 and cyclohexyl), 1.39–1.28 (m, 6H, SnBu3), 1.28–1.10 (m, 5H, cyclohexyl), 1.09–0.85 (m, 15H, SnBu3). 13C NMR (100.6 MHz, CDCl3): δ 152.3, 138.2, 128.8, 128.4, 125.5, 104.9, 63.7, 58.2, 57.6, 44.8, 33.6, 31.7, 31.2, 29.2, 27.5, 26.1, 25.2, 25.1, 13.7, 11.0. EI-LRMS, m/z (%): 520 (0.2) [M+–C4H9], 518 (0.19), 176 (100), 164 (82). Elemental analysis calcd (%) for C30H51NSSn (576.5): C 62.50, H 8.92, N 2.43; found C 62.56, H 8.86, N 2.48.
3.3.4 1-Cyclohexyl-3-(2,2-diphenyl-1-phenylthio-2-hydroxyethyl)-4-methylenepyrrolidine 8d
From amine 6a (0.79 g, 84%). Mp = 151–153 °C. Data from major diastereoisomer: 1H NMR (400 MHz, CDCl3): δ 8.45 (s broad, 1H, OH), 7.67 (d, J = 7.2 Hz, 4H, ArH), 7.40–7.06 (m, 11H, ArH), 4.81–4.77 (m, 1H, =CHH), 4.72 (d, J = 4.8 Hz, 1H, CHCHS), 4.55–4.51 (m, 1H, =CHH), 3.83 (d, J = 9.2 Hz, 1H, NCHHCH), 3.24–3.17 (m, 1H, CHCHS), 2.82 (d, J = 14.0 Hz, 1H, NCHHC=), 2.63 (d, J = 14.0 Hz, 1H, NCHHC=), 2.30 (dd, J = 9.2 and 7.2 Hz, 1H, NCHHCH), 2.16–1.22 (m, 11H, CHN and 5 × CH2 cyclohexyl). 13C NMR (100.6 MHz, CDCl3): δ 147.8, 147.7, 146.5, 136.4, 131.6, 128.8, 127.6, 127.0, 126.7, 126.6, 126.2, 125.8, 125.2, 108.6, 79.5, 62.6, 62.2, 56.7, 53.6, 47.6, 31.2, 31.0, 25.7, 24.9, 24.8. EI–LRMS, m/z (%): 359 (9) [M+–PhSH], 163 (100). Elemental analysis calcd (%) for C31H35NOS (469.7): C 79.27, H 7.51, N 2.98; found C 79.21, H 7.43, N 2.90.
3.3.5 1-Cyclohexyl-3-(1-deuterio-1-trimethylsilylmethyl)-4-methylenepyrrolidine 8e
From amine 6b (0.45 g, 90%). Rf = 0.19 (hexane/AcOEt, 5:1). 1H NMR (400 MHz, CDCl3): δ 4.87–4.84 (m, 1H, =CHH), 4.80–4.77 (m, 1H, =CHH), 3.64 (d, J = 14.0 Hz, 1H, NCHHC=), 3.21 (t, J = 8.0 Hz, 1H, NCHHCH), 2.90 (ddd, J = 14.0, 4.8 and 2.4, 1H, NCHHC=), 2.68–2.57 (m, 1H, CHCHD), 1.97–1.83 (m, 4H, NCHHCH, NCH and cyclohexyl), 1.77–1.54 (m, 3H, cyclohexyl), 1.28–1.05 (m, 5H, cyclohexyl), 0.96–0.92 (m, 1H, CHD), –0.01 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 155.2, 103.1, 63.8, 59.6, 57.1, 38.9, 31.7, 31.6, 26.0, 25.0, 24.9, 19.7 (t, J = 18.4 Hz), –0.9. EI–LRMS, m/z (%): 252 (14) [M+], 209 (100), 73 (57). Elemental analysis calcd (%) for C15H28DNSi (252.5): C 71.35, H/D 11.98, N 5.55; found C 71.27, H/D 12.05, N 5.63.
3.3.6 3-[1,1Bis(trimethylsilyl)methyl]-1-cyclohexyl-4-methylenepyrrolidine 8f
From amine 6b (0.52 g, 80%). Rf = 0.33 (hexane/AcOEt, 4:1). 1H NMR (400 MHz, CDCl3): δ 4.86–4.82 (m, 1H, =CHH), 4.76–4.72 (m, 1H, =CHH), 3.67 (d, J = 14.8 Hz, 1H, NCHHC=), 3.16 (t, J = 8.0 Hz, 1H, NCHHCH), 2.99–2.90 (m, 2H, NCHHC= and CHCHSi2), 2.15 (dd, J = 10.4 and 8.0 Hz, 1H, NCHHCH), 2.02–1.53 (m, 6H, NCH and cyclohexyl), 1.30–1.05 (m, 5H, cyclohexyl), 0.39 (d, J = 1.2 Hz, 1H, CHSi2), 0.01 (s, 9H, 3 × CH3 SiMe3), 0.00 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 154.3, 103.2, 63.7, 57.6, 57.2, 41.4, 31.6, 31.5, 26.0, 25.1, 25.0, 16.6, 2.5, 0.7. EI–LRMS, m/z (%): 323 (5) [M+], 322 (8), 151 (100), 73 (90). Elemental analysis calcd (%) for C18H37NSi2 (323.7): C 66.80, H 11.52, N 4.33; found C 66.71, H 11.47, N 4.39.
3.3.7 1-Cyclohexyl-3-(1-phenylthio-1-trimethylsilylmethyl)-4-methylenepyrrolidine 8g
From amine 6b (0.61 g, 85%). Data from minor diastereoisomer: Rf = 0.5 (hexane/AcOEt, 4:1). 1H NMR (400 MHz, CDCl3): δ 7.37–7.32 (m, 2H, ArH), 7.28–7.23 (m, 2H, ArH), 7.16–7.11 (m, 1H, ArH), 4.94–4.91 (m, 1H, =CHH), 4.86–4.83 (m, 1H, =CHH), 3.32 (d, J = 13.6 Hz, 1H, NCHHC=), 3.27–3.20 (m, 1H, NCHHC=), 3.11–3.03 (m, 1H, CHCHSSi), 2.95 (dd, J = 8.8 and 7.2 Hz, 1H, NCHHCH), 2.80 (d, J = 3.6 Hz, 1H, CHCHSSi), 2.62 (dd, J = 8.8 and 6.4 Hz, 1H, NCHHCH), 2.02–1.85 (m, 3H, NCH and cyclohexyl), 1.77–1.55 (m, 3H, cyclohexyl), 1.30–1.10 (m, 5H, cyclohexyl), 0.19 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 151.5, 137.3, 129.0, 128.9, 125.8, 105.0, 63.6, 58.3, 55.6, 45.2, 37.8, 31.6, 31.4, 26.0, 25.0, 24.9, –0.2. EI-LRMS, m/z (%): 359 (0.16) [M+], 164 (100). Elemental analysis calcd (%) for C21H33NSSi (359.6): C 70.13, H 9.25, N 3.89; found C 70.19, H 9.18, N 3.98. Data from major diastereoisomer: Rf = 0.35 (hexane/AcOEt, 1:1). 1H NMR (400 MHz, CDCl3): δ 7.38–7.34 (m, 2H, ArH), 7.20–7.14 (m, 2H, ArH), 7.12–7.07 (m, 1H, ArH), 4.82–4.79 (m, 1H, =CHH), 4.66–4.63 (m, 1H, =CHH), 3.62 (d, J = 13.2 Hz, 1H, NCHHC=), 3.23–3.10 (m, 2H, NCHHCH and CHCHSSi), 2.88 (ddd, J = 13.2, 4.8 and 2.8 Hz, 1H, NCHHC=), 2.82 (d, J = 2.8 Hz, 1H, CHCHSSi), 2.37 (dd, J = 9.2 and 8.4 Hz, 1H, NCHHCH), 2.10–2.00 (m, 1H, NCH), 1.98–1.55 (m, 5H, cyclohexyl), 1.32–1.07 (m, 5H, cyclohexyl), 0.12 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 150.3, 139.0, 130.3, 128.4, 125.8, 105.4, 63.6, 58.3, 55.3, 43.9, 37.9, 31.6, 31.5, 26.0, 25.0, 24.9, -1.7. EI-LRMS, m/z (%): 359 (0.16) [M+], 164 (100). Elemental analysis calcd (%) for C21H33NSSi (359.6): C 70.13, H 9.25, N 3.89; found C 70.18, H 9.35, N 3.80.
3.3.8 1-Cyclohexyl-3-methylene-4-(1-tributyltin-1-trimethylsilylmethyl)pyrrolidine 8h
From amine 6b (0.79 g, 73%). Data from minor diastereoisomer: Rf = 0.22 (hexane/AcOEt, 10:1). 1H NMR (400 MHz, CDCl3): δ 4.91–4.87 (m, 1H, =CHH), 4.81–4.78 (m, 1H, =CHH), 3.70 (d, J = 13.2 Hz, 1H, NCHHC=), 3.21–2.95 (m, 3H, NCHHC=, CHCHSiSn and NCHHCH), 2.20–0.68 (m, 40H, CHCHSiSn, NCH, NCHHCH, 5 × CH2 cyclohexyl and 3 × (CH2)3CH3 SnBu3), 0.02 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 155.0, 104.5, 63.9, 59.5, 57.8, 43.0, 31.6, 31.5, 29.2, 27.6, 26.0, 25.1, 25.0, 13.8, 13.7, 10.9, 2.1. EI–LRMS, m/z (%): 485 (1), 483 [M+–C4H9] (0.6), 250 (100). Elemental analysis calcd (%) for C27H55NSiSn (540.5): C 59.99, H 10.26, N 2.59; found C 60.10, H 10.31, N 2.51. Data from major diastereoisomer: Rf = 0.21 (hexane/AcOEt, 10:1). 1H NMR (400 MHz, CDCl3): δ 4.90–4.85 (m, 1H, =CHH), 4.80–4.76 (m, 1H, =CHH), 3.72 (d, J = 14.4 Hz, 1H, NCHHC=), 3.27 (t, J = 8.0 Hz, 1H, NCHHCH), 3.20–3.10 (m, 1H, CHCHSiSn), 3.00–2.93 (m, 1H, NCHHC=), 2.00–0.68 (m, 40H, CHCHSiSn, NCH, NCHHCH, 5 × CH2 cyclohexyl and 3 × (CH2)3CH3 SnBu3), 0.03 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 155.1, 103.5, 63.8, 60.3, 57.0, 42.5, 31.6, 31.5, 29.2, 27.6, 26.0, 25.1, 25.0, 15.3, 13.6, 11.6, 0.5. EI-LRMS, m/z (%): 483 (5) [M+–C4H9], 248 (100). Elemental analysis calcd (%) for C27H55NSiSn (540.5): C 59.99, H 10.26, N 2.59; found C 60.12, H 10.33, N 2.50.
3.3.9 1-Cyclohexyl-3-(1-deuterio-1-tributyltinmethyl)-4-methylenepyrrolidine 8i
From amine 6c (0.88 g, 94%). Rf = 0.32 (hexane/AcOEt, 5:1). 1H NMR (400 MHz, CDCl3): δ 4.89–4.87 (m, 1H, =CHH), 4.82–4.80 (m, 1H, =CHH), 3.62 (d, J = 14.0 Hz, 1H, NCHHC=), 3.14 (t, J = 8.0 Hz, 1H, NCHHCH), 2.98 (ddd, J = 14.0, 4.8 and 2.0 Hz, 1H, NCHHC=), 2.86–2.77 (m, 1H, CHCHDSn), 1.97–1.83 (m, 40H, 5 × CH2 cyclohexyl, NCH, 3 × (CH2)3CH3 SnBu3, NCHHCH and CHCHDSn). 13C NMR (100.6 MHz, CDCl3): δ 155.6, 103.5, 63.8, 60.9, 57.6, 40.9, 31.7, 31.6, 29.2, 27.4, 26.0, 25.0, 24.9, 13.7, 12.0 (t, J = 19.8 Hz), 9.4. Elemental analysis calcd (%) for C24H46DNSn (469.4): C 61.42, H/D 10.31, N 2.98; found C 61.91, H/D 10.39, N 2.91.
3.3.10 3-(1-Deuterio-1-trimethylsilylmethyl)-4-methylene-1-phenylpyrrolidine 8j
From amine 6d (0.26 g, 53%). Mp = 43–45 °C. 1H NMR (400 MHz, CDCl3): δ 7.33 (dd, J = 8.4 and 7.6 Hz, 2H, ArH), 6.80 (t, J = 7.6 Hz, 1H, ArH), 6.66 (d, J = 8.4, 2H, ArH), 5.06–5.03 (m, 1H, =CHH), 5.02–4.99 (m, 1H, =CHH), 4.15 (d, J = 13.6 Hz, 1H, NCHHC=), 3.92 (dd, J = 13.6 and 1.6 Hz, 1H, NCHHC=), 3.75 (t, J = 6.8 Hz, 1H, NCHHCH), 3.00–2.88 (m, 2H, NCHHCH and CHCHDSi), 1.07 (s, 1H, CHD), 0.72 (d, J = 10.4 Hz, 1H, CDH), 0.18 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 152.7, 147.7, 129.1, 116.1, 111.8, 104.3, 55.1, 52.8, 39.0, 18.6 (t, J = 18.7 Hz), –0.9. EI–LRMS, m/z (%): 246 (75) [M+], 173 (56), 158 (100), 73 (84). Elemental analysis calcd (%) for C15H22DNSi (246.4): C 73.10, H/D 9.82, N 5.68; found C 73.15, H/D 9.75, N 5.74.
3.3.11 3-Methylene-1-phenyl-4-(1-phenylthio-1-trimethylsilylmethyl)pyrrolidine 8k
From amine 6d (0.35 g, 50%). Data from minor diastereoisomer: Rf = 0.16 (hexane/AcOEt, 25:1). 1H NMR (400 MHz, CDCl3): δ 7.45–7.41 (m, 2H, ArH), 7.36–7.19 (m, 5H, ArH), 6.79 (t, J = 7.4 Hz, 1H, ArH), 6.67 (d, J = 8.8 Hz, 2H, ArH), 5.15–5.12 (m, 1H, =CHH), 5.07–5.03 (m, 1H, =CHH), 4.07 (dd, J = 14.0 and 2.0 Hz, 1H, NCHHC=), 3.87 (d, J = 14.0 Hz, 1H, NCHHC=), 3.55–3.47 (m, 2H, NCHHCH), 3.31 (m, 1H, CHCHSSi), 2.91 (d, J = 4.0 Hz, 1H, CHCHSSi), 0.20 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 149.0, 147.7, 138.5, 130.2, 129.1, 128.5, 126.0, 116.5, 112.3, 107.0, 53.6, 51.8, 44.2, 38.2, -1.5. EI–LRMS, m/z (%): 353 (0.15) [M+], 158 (100). Elemental analysis calcd (%) for C21H27NSSi (353.6): C 71.33, H 7.70, N 3.96; found C 71.25, H 7.80, N 3.85. Data from major diastereoisomer: Rf = 0.13 (hexane/AcOEt, 25:1). 1H NMR (400 MHz, CDCl3): δ 7.42–7.38 (m, 2H, ArH), 7.33–7.15 (m, 5H, ArH), 6.78 (t, J = 7.4 Hz, 1H, ArH), 6.63 (d, J = 8.8 Hz, 2H, ArH), 5.00–4.97 (m, 1H, =CHH), 4.93–4.90 (m, 1H, =CHH), 4.02 (d, J = 14.0 Hz, 1H, NCHHC=), 3.85 (dd, J = 14.0 and 2.0 Hz, 1H, NCHHC=), 3.70–3.60 (m, 1H, NCHHCH), 3.46–3.36 (m, 2H, NCHHCH and CHCHSSi), 2.94 (d, J = 2.8 Hz, 1H, CHCHSSi), 0.25 (s, 9H, 3 × CH3 SiMe3). 13C NMR (100.6 MHz, CDCl3): δ 149.0, 147.7, 138.5, 130.2, 129.1, 128.5, 126.0, 116.5, 112.3, 107.0, 53.6, 51.8, 44.2, 38.2, –1.5. EI–LRMS, m/z (%): 353 (0.15) [M+], 158 (100). Elemental analysis calcd (%) for C21H27NSSi (353.6): C 71.33, H 7.70, N 3.96; found C 71.24, H 7.82, N 3.90.
3.3.12 3-Methylene-1-phenyl-4-(1-tributyltin-1-trimethylsilylmethyl)pyrrolidine 8l
From amine 6d (0.59 g, 55%). Mixture of diastereoisomers: Rf = 0.24 (hexane). 1H NMR (400 MHz, CDCl3): δ 7.37–7.31 (m, 2H, ArH), 6.85–6.79 (m, 1H, ArH), 6.70–6.65 (m, 2H, ArH), 5.20–5.15 (m, 1H, =CHH), 5.08–5.04 (m, 1H, =CHH), 4.26–4.16 (m, 2H, NCHHC=, major diast.), 4.05–3.93 (m, 2H, NCHHC=, minor diast.), 3.87 (t, J = 8.8 Hz, 1H, NCHHCH, major diast.), 3.71 (t, J = 8.8 Hz, 1H, NCHHCH, minor diast.), 3.56–3.47 (m, 1H, CHCHSiSn, major diast.), 4.47–3.38 (m, 1H, CHCHSiSn, minor diast.), 3.13 (t, J = 8.8 Hz, 1H, NCHHCH, minor diast.), 2.86 (t, J = 8.8 Hz, 1H, NCHHCH, major diast.), 1.70–1.30 (m, 13H, CHCHSiSn and SnBu3), 1.12–0.80 (m, 15H, SnBu3), 0.22 (s, 9H, 3 × CH3 SiMe3, major diast.), 0.15 (s, 9H, 3 × CH3 SiMe3, minor diast.). 13C NMR (100.6 MHz, CDCl3): δ 153.4, 153.2, 147.7, 147.4, 129.1, 116.4, 116.3, 112.1, 111.9, 105.5, 104.4, 56.4, 55.7, 53.6, 52.9, 43.0, 41.9, 29.3, 29.2, 27.6, 27.5, 15.7, 14.4, 13.7, 13.6, 11.4, 11.0, 2.0, 0.4. EI–LRMS, m/z (%): major diast.: 477 (5) [M+-C4H9], 242 (100); minor diast.: 477 (1.8) [M+-C4H9], 242 (100). Elemental analysis calcd (%) for C27H49NSiSn (534.5): C 60.67, H 9.24, N 2.62; found C 60.78, H 9.29, N 2.57.
3.3.13 3-(1-Deuterio-1-phenylmethyl)-4-methylene-1-phenylpyrrolidine 8m
From amine 6e (0.31 g, 61%). Rf = 0.38 (hexane/AcOEt, 10:1). 1H NMR (200 MHz, CDCl3): δ 7.53–6.73 (m, 10H, ArH), 5.24 and 5.13 (2s, 2H, =CHH and =CHH), 4.15 (s, 2H, NCH2C=), 3.55 (dd, J = 7.0 and 6.7 Hz, 1H, NCHHCH), 3.32–3.08 (m, 3H, NCHHCH, CH and CHD). 13C NMR (50.5 MHz, CDCl3): δ 149.5, 147.7, 139.9, 129.0, 128.7, 128.3, 126.1, 116.3, 112.0, 105.8, 53.1, 52.9, 44.2, 38.8 (t, J = 20.3 Hz). EI-LRMS, m/z (%): 250 (20) [M+], 249 (18), 158 (100), 156 (37).
3.3.14 (2R*,7aR*)-1-Cyclohexyl-3-phenylthio-2,3,5,6,7,7a-hexahydro-1H-indole 12a
From amine 10 (0.60 g, 92%). Rf = 0.32 (hexane/AcOEt, 5:1). 1H NMR (400 MHz, CDCl3): δ 7.35–7.30 (m, 2H, ArH), 7.28–7.22 (m, 2H, ArH), 7.18–7.12 (m, 1H, ArH), 5.54–5.50 (m, 1H, =CH), 3.22 (dd, J = 8.8 and 7.2 Hz, 1H, NCHHCH), 3.16–3.11 (m, 1H, CHCHHS), 3.08–3.00 (m, 1H, NCHC=), 2.89–2.77 (m, 2H, CHCHHS), 2.71–2.61 (m, 1H, NCH), 2.32 (dd, J = 8.8 and 8.0 Hz, 1H, NCHHCH), 1.85–0.99 (m, 16H, 5 × CH2 cyclohexyl and =CHCH2CH2CH2). 13C NMR (100.6 MHz, CDCl3): δ 143.2, 136.4, 129.1, 128.7, 125.8, 119.9, 58.3, 56.6, 51.9, 39.2, 39.1, 32.1, 28.1, 26.4, 25.7, 25.1, 24.1, 20.3. EI–LRMS, m/z (%): 327 (3) [M+], 204 (100), 176 (78). Elemental analysis calcd (%) for C21H29NS (327.5): C 77.01, H 8.92, N 4.28; found C 77.10, H 9.04, N 4.21.
3.3.15 (2R*,7aR*)-3-[1,1-Bis(phenylthio)methyl]-1-cyclohexyl-2,3,5,6,7,7a-hexahydro-1H-indole 12b
From amine 10 (0.78 g, 90%). Rf = 0.32 (hexane/AcOEt, 5:1). 1H NMR (400 MHz, CDCl3): δ 7.44–7.36 (m, 4H, ArH), 7.29–7.18 (m, 6H, ArH), 5.63–5.59 (m, 1H, =CH), 4.55 (d, J = 4.0 Hz, 1H, CHCHS2), 3.31–3.20 (m, 2H, NCHHCH, NCHC=), 3.18 (t, J = 8.4 Hz, 1H, NCHHCH), 2.77 (dd, J = 8.4 and 7.6 Hz, 1H, NCHHCH), 2.73–2.67 (m, 1H, NCH), 2.15–0.81 (m, 16H, 5 × CH2 cyclohexyl and =CHCH2CH2CH2). 13C NMR (100.6 MHz, CDCl3): δ 141.2, 135.1, 134.7, 132.6, 132.3, 128.9, 128.8, 127.6, 127.4, 121.4, 63.8, 59.3, 56.8, 48.6, 44.4, 32.0, 28.3, 26.4, 25.8, 25.2, 24.3, 20.2. Elemental analysis calcd (%) for C27H33NS2 (435.7): C 74.43, H 7.63, N 3.21; found C 74.48, H 7.55, N 3.25.
Acknowledgements
Financial support from the Ministerio de Ciencia y Tecnología (MCYT, BQU2001–1079) and Junta de Castilla y León (BU24/02) is gratefully acknowledged. Y.F. thanks Junta de Castilla y León for a fellowship.


