1 Introduction
It is well known that the structural chemistry of vanadium is characterised by an exceptional diversity. Indeed, the vanadium in the solid state gives currently rise to the oxidation states +3 up to +5, each one dealing with different coordinations. In the large domain of the mixed organic/inorganic compounds, the hydrothermal way favours the synthesis of solids with infinite layers [1] or even three-dimensional frameworks [2], whereas condensation processes at room temperature rather lead to molecular species [3]. In alkaline medium, the V5+ ions provide tetra, penta and decavanadate species, but the works of M. Pope and A. Müller especially have allowed to extend this chemistry to an important number of molecular species with VIV/VV mixed valence which exhibit very different features. Their structures are (i) compact as [V13O34]3– [4], (ii) hollow and templated by anionic (halide, chlorate, phosphate, e.g.) or neutral entities in [V15O36]5–, [V18O42]12– [5] or [V22O54]8– [6], or (iii) open as exemplified by [V12O32]4– [7]. Among this large class of materials, the V16 stoichiometry is scarcely encountered. To our knowledge, it is just reported V16 species whose structures are build up from the assembling of smaller units (two octanuclear units in [8] or two hexa and one tetranuclear oligomers in [9] fused by oxo anions).
This paper deals with the synthesis and the structure characterisation of a new polyoxovanadate molecular entity whose spherical ball-shaped architecture is build up from 16V5+ polyhedra. We apologize to Francis Sécheresse for failing in our attempt to give a rugby ball shape to this new polyoxovanadate moiety.
2 Experimental
2.1 Synthesis
The synthesis of the title compound was achieved in a closed flask at room temperature from a mixture of VOSO4·5H2O (Prolabo), 1,4-benzenedicarboxylic acid (Aldrich, 99.8%) and N,N-dimethylformamide (Aldrich, 99.8%) in the molar ratio 1:2:117. After several weeks, thin green hexagonal platelets grew which then agglomerated into large blocks. It is worth noting that (i) these crystals are quickly destroyed after some minutes outside of the medium synthesis, (ii) the same mixture hydrothermally treated at 473 K leads to the three-dimensional microporous vanado(III)carboxylate MIL-68 already described [10]. This last point justifies the presence of the terephthalic acid in the medium. Maybe, this acid acts as a catalyst for the hydrolysis of DMF into dimethylammonium ions. The very poor stability of the crystal outside of the mother liquor prohibits a lot of characterisation methods (elemental analysis, thermal analysis…).
2.2 Structure determination
One monocrystalline fragment was glued on a glass fibre with paratone then quickly aligned on a Brüker X8 APEX2 CCD diffractometer operating at 100 K. The intensities were recorded using graphite monochromatic Mo(Kα) = 0.71073 Å wavelength. The data were reduced and corrected from Lorentz-Polarization and absorption effects with the SADABS program (G. Sheldrick, version 2.10 unpublished). The structure was solved by applying the direct methods of SHELXTL-V6.12 (TREF option), the vanadium, sulphur and oxygen atoms were first located then the remaining atoms were deduced from Fourier difference maps. All atoms except hydrogen (found using geometrical constraints) were anisotropically refined. The contribution to the intensities of one fully disordered DMF molecule was corrected using the PLATON/SQUEEZE program [11] in view to improve the quality of the structural model of the polyoxovanadate species.
The details for the data collection as well as the principal bond lengths are summarised in Tables 1 and 2, respectively.
Details of data collection and principal crystallochemical data for [(SO4) ⊂ V16O42]·(SO4)2((CH3)2NH2)10((CH3)2NCHO)4
| Empirical formula | C32H108N14O58S3V16 |
| Formula weight | 2528 g.mol–1 |
| Temperature | 100(2) K |
| Wavelength | 0.71073 Å |
| Crystal system | monoclinic |
| Space group | C2/c (No. 15) |
| Lattice parameters | a = 25.554(6) Å |
| b = 14.227(3) Å | |
| c = 28.485(6) Å | |
| β = 113.21(1)° | |
| Volume, Z | 9518(4) Å3, 4 |
| Density (Calc.) | 1.765 g.cm–3 |
| Absorption coefficient | 16.55 cm–1 |
| F(000) | 5112 |
| Crystal size | 0.120 × 0.100 × 0.060 mm |
| θ range for data collection | 1.56–30.00° |
| Limiting indices | –35 ≤ h ≤ 35 |
| –20 ≤ k ≤ 12 | |
| –40 ≤ l ≤ 32 | |
| Reflections collected | 55 683 |
| Independent reflections | 13,877 [R(int) = 0.0366] |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 13 877/0/560 |
| Goodness-of-fit on F2 | 1.082 |
| Final R indices [I > 2σ(I)] | R1 = 0.0434, wR2 = 0.1269 |
| Extinction coefficient | none |
| Largest difference peak and hole | 2.337 and –1.008 e Å–3 |
Principal bond lengths (Å) in each [(SO4) ⊂ V16O42]6– polyoxovanadate moiety. In italic, the valence bond (u.v.) for the vanadium and the sulphur atoms
| V(1)–O(1) | 1.615(2) | V(4)–O(12) | 1.618(2) | V(7)–O(19) | 1.616(2) |
| V(1)–O(2) | 1.732(2) | V(4)–O(13) | 1.730(2) | V(7)–O(20) | 1.7910(7) |
| V(1)–O(3) | 1.881(2) | V(4)–O(8) | 1.878(2) | V(7)–O(16) | 1.916(2) |
| V(1)–O(4) | 1.903(2) | V(4)–O(14) | 1.911(2) | V(7)–O(2) | 1.935(2) |
| V(1)–O(5) | 2.066(2) | V(4)–O(11) | 2.065(2) | V(7)–O(10) | 1.964(2) |
| 4.938 | 4.924 | 4.775 | |||
| V(2)–O(6) | 1.607(2) | V(5)–O(15) | 1.606(2) | V(8)–O(21) | 1.616(2) |
| V(2)–O(7) | 1.763(2) | V(5)–O(16) | 1.749(2) | V(8)–O(22) | 1.7872(7) |
| V(2)–O(8) | 1.938(2) | V(5)–O(3) | 1.933(2) | V(8)–O(13) | 1.919(2) |
| V(2)–O(5) | 1.983(2) | V(5)–O(11) | 1.987(2) | V(8)–O(7) | 1.943(2) |
| V(2)–O(4) | 1.989(2) | V(5)–O(14) | 2.007(2) | V(8)–O(18) | 1.964(2) |
| 4.726 | 4.748 | 4.765 | |||
| V(3)–O(9) | 1.612(2) | V(6)–O(17) | 1.617(2) | ||
| V(3)–O(10) | 1.727(2) | V(6)–O(18) | 1.728(2) | sulphate group | |
| V(3)–O(4) | 1.902(2) | V(6)–O(14) | 1.893(2) | S(1)–O(23) | 1.483(2) (2×) |
| V(3)–O(11) | 1.902(2) | V(6)–O(5) | 1.907(2) | S(1)–O(24) | 1.486(2) (2×) |
| V(3)–O(8) | 2.041(2) | V(6)–O(3) | 2.039(2) | 5.768 | |
| 4.960 | 4.945 |
2.3 IR spectroscopy
IR spectrum (cm–1) of [(SO4) ⊂ V16O42]·(SO4)2((CH3)2NH2)10((CH3)2NCHO)4 in KBr pellets was recorded on a FTIR Nicolet Magna-550 spectrophotometer.
Bands attributed to the [(SO4) ⊂ V16O42]6– species: (ν S–O): 1116 (s), 1020 (m), (ν V=O) [12,13]: 980 (sh) and 956 (s), (ν V–O–V): 796 (m), 620 (m), 584 (m) , 516 (w), 490 (w), and 447 (w).
Bands attributed to the dimethylammonium and DMF organic species: 3434 (br), 3055 (br), (ν C–H): 2964 (w), 2921 (w), and 2857 (w), 2777 (br), 1629 (m), 1464 (m), 1431 (w), 1403 (vw), 1383 (vw), 1251 (w).
The bands due to the NH2 groups of the dimethylammonium ions are not unambiguously showed. Indeed, the presence of residual water in KBr gives rise to a broad band at 3434 cm–1, in the range expected for the N–H vibrators.
3 Discussion
[(SO4) ⊂ V16O42]·(SO4)2((CH3)2NH2)10((CH3)2NCHO)4 exhibits a molecular structure built up from discrete [(SO4) ⊂ V16O42]6– species between them are interleaved free sulphate groups, disordered DMF molecules and dimethylammonium ions which neutralise the negative charges of the polyoxovanadate moieties. From the observation of the residual electronic density calculated by Fourier difference syntheses, It was assumed that the DMF solvent was partially degraded to give rise to dimethylammonium ions. Indeed, this is the only possibility being the absence of alkaline or alkaline-earth ions in the medium synthesis, to obtain the positive ions necessary to neutralise the electrical charges of the polyanions. Moreover, the geometric characteristics of the ions deduced from the structure determination are in perfect agreement with those of dimethylammonium units. Six independent DMA ions were localised then refined with a fixed 5/6 multiplicity for electroneutrality requirements.
The polyanions correspond to [(SO4) ⊂ V16O42]6– polyoxovanadates built up from the connection by edges and vertices of 16VVO5 square pyramids (Fig. 1). Both the geometry of the polyhedra (three V–O distances slightly smaller than 2 Å and one around 1.7 Å in the basal plane with one short ~1.6 Å vanadyl linkage perpendicularly disposed) and the valence bonds calculations [14] demonstrate that all the vanadium ions are in the +5 oxidation state (Table 2). The polyanion is itself built up from two sub-units (Fig. 2): one 12-membered ring of edge-shared square pyramids with all the terminal vanadyl bonds pointing outside the ring and two dimers of square pyramids sharing one oxo anion. Each ring whose diameter is close to 7 Å (V–V distances), is vertices-capped on both sides by one dimer, each dimer sharing all the vertices of the basal planes of the two square pyramids. The resulting polyoxovanadate moiety exhibits a spherical shape with 16 terminal vanadyl linkages pointing on the outer surface of the ball. Inside the sphere is encapsulated one tetrahedral sulphate group whose oxygen atoms are slightly bonded to some of the vanadium atoms (V–O > 2.45 Å). Consequently, the coordination of these vanadium atoms could be described as very elongated octahedra, the long distance being trans-located to the short vanadyl linkage.
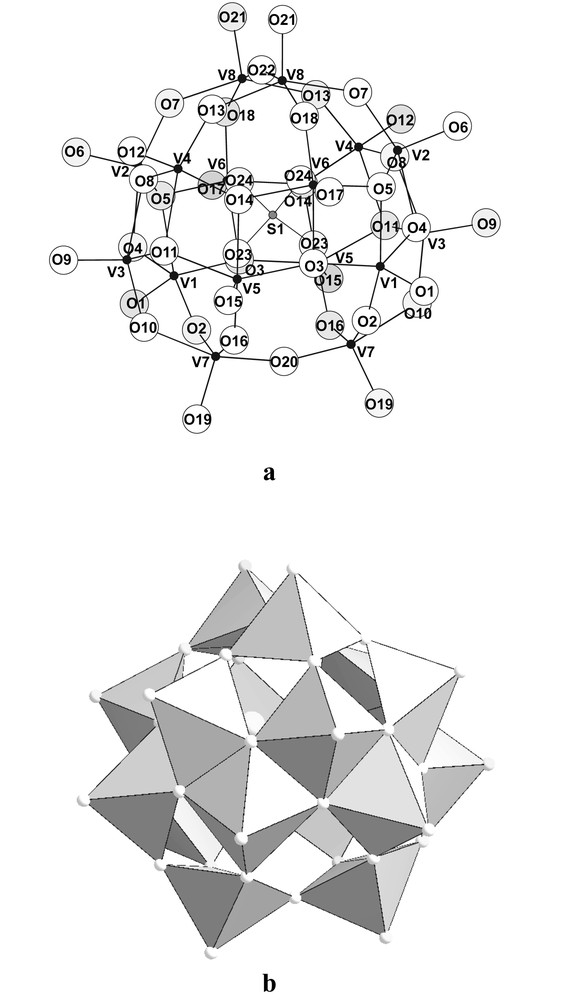
(a) ‘Balls and Sticks’ and (b) polyhedral representations of one [(SO4) ⊂ V16O42]6– entity.

View of one [V16O42]4– polyoxovanadate moiety showing the two types of building sub-units: the 12-membered ring (polyhedra) capped by the two dimers (‘Balls and Sticks’ representation, dashed and full lines for the dimers below and above the ring, respectively). Dark grey circles for the vanadium atoms.
It is worth noting that the 12-membered rings show some structural relation-ships with the parental vanadium oxides V2O5 and V3O7. Indeed, this ring can be thought as the result of the cyclisation of one linear ribbon of 12 edge-shared square pyramids. This ribbon (Fig. 3a) is well-known in the chemistry of the vanadium oxides, it was labelled {UU} chains by Zavalij and Whittingham [1] relatively to the up-position of all the vanadyl linkages; it is a constitutive building block of the three-dimensional structure of V3O7 [15]. In an another hand, V2O5 [16] presents a two-dimensional structure whose layers are built up from the connection of closely related ribbons of edge-shared square pyramids. In this case, they are labelled {UD} chains since the vanadyl linkages alternatively point up and down the middle plane of the chains.
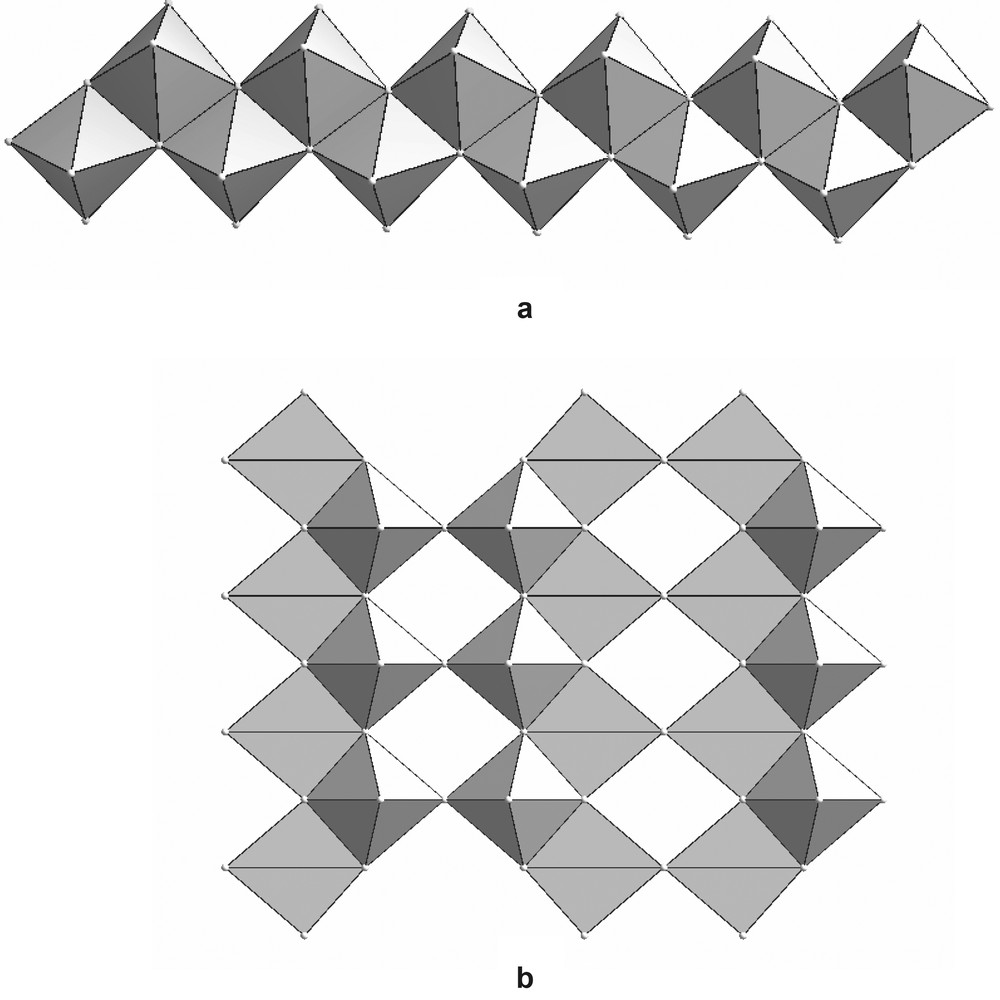
(a) {UU} chain of VO5 square pyramids as encountered in V3O7, and (b) one layer of V2O5 build up from the vertices-connections of {UD} chains of VO5 square pyramids.
Finally, this new [V16O42]4– discrete entity shows the curious feature to give rise to structural overlaps between the two very different families of polyoxovanadates and extended vanadium oxides structures.
An ORTEP drawing of the polyanion, the IR spectrum and the complete cif file are given as supplementary material. Furthermore, the cif file corresponding to the structure determination of [(SO4) ⊂ V16O42]·(SO4)2((CH3)2NH2)10((CH3)2NCHO)4 has been deposited with the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK and can be obtained under No. 242770 by contacting the CCDC.


