1 Introduction
The crystal engineering of coordination polymers is currently of great interest due to interesting host–guest chemistry and catalytic, magnetic, and electrical conductivity properties of these materials [1–8].
One of the synthetic strategies for preparation of metal–organic polymers is based on bonding of metal cations by bi- or poly-hapto organic ligands (linkers) to networks of various dimensionalities. Using different linkers, metal–organic polymers can be deliberately designed to have micropores and/or large open channels of desirable size. Combining covalent (coordination bond) and π–π stacking or H-bonding (interligand) interactions to build the channel walls offers the advantage of reversible opening/closing of these channels. Such hybrid coordination polymers are soluble and can be even spun into nanofibers [9,10]. One of the most popular linkers is a rigid bidentate bridging ligand, 4,4′-bipyridine, which allows to obtain widely spaced transition/post-transition metal centers in polymeric compounds. A number of 4,4′-bipyridine based networks have been reported, including 1D chains [11], 2D grids [12–14] and 3D frameworks [15,16].
Herein we report synthesis and crystal structure of a new framework metal–organic polymer [Fe(4,4′-bpy)3(H2O)2](PF6)2·2(4,4′-bpy)·5 H2O (1). It was obtained by using 4,4′-bipyridine as a linker for Fe(II) cations.
2 Experimental section
2.1 Synthesis of 1
Method 1. To a solution of FeSO4·7 H2O (0.20 g, 0.719 mmol) in 10 ml of water a hot solution of 4,4′-bipyridine (C10H8N2) (0.56 g, 3.59 mmol) in 6 ml of water was added. The reaction mixture was heated (70–80 °C) for 30 min, and a solution of KPF6 (0.50 g, 2.71 mmol) in 5 ml of water was added. A yellow precipitate appeared was filtered out, and the filtrate was left to crystallize. After 4 days yellow rhombic crystals of 1 were collected by filtration and dried on filter paper. Yield: 0.77 g (86%).
Elemental analysis for C50H54F12FeN10O7P2 Calc. (%): C 47.94; H 4.34; N 11.18; Found (%): C 47.63; H 4.22; N 10.97.
IR (KBr pellet, cm–1): 3618 (sh); 3525 (sh); 3447 (sh); 1601 (m); 1539 (sh); 1485 (sh); 1407 (m); 1313 (sh); 1228 (sh); 1072 (m); 994 (sh); 841 (s); 807 (m); 729 (sh); 620 (m); 558 (m); 496 (sh).
Method 2. To a warm solution of [Fe3O(CH3COO)6(H2O)3] (0.20 g, 0.34 mmol) in 25 ml of water a hot solution of 4,4′-bipyridine (0.16 g, 1.02 mmol) in 8 ml of water was added. The reaction mixture was stirred for 30 min under ambient temperature and a solution of KPF6 (0.12 g, 0.65 mmol) in 5 ml of water was added. Red amorphous precipitate appeared was filtered out, and the filtrate was left to crystallize. After 7 days yellow rhombic crystals of 1 formed. Those were collected by filtration and dried on filter paper. Yield: 0.119 g (84% based on Fe2+). Correct IR spectrum and C, H, N analysis.
2.2 X-ray structure determination
The crystallographic data for 1 and X-ray experimental data are listed in Table 1. The structure was solved by direct methods and refined by the full-matrix least-squares method [17,18]. All non-hydrogen atoms were refined anisotropically. Hydrogen atoms, excluding water hydrogens, were introduced in their calculated positions.
Crystallographic data and details of diffraction experiments for 1
| Empirical formula | C50H54F12FeN10O7P2 |
| Formula weight | 1252.82 |
| Crystal system | Monoclinic |
| Space group | Cc |
| a (Å) | 18.290(4) |
| b (Å) | 11.666(2) |
| c (Å) | 27.084(5) |
| β (°) | 108.21(3) |
| V (Å3) | 5489.2(19) |
| Z | 4 |
| Dcalc (g cm–3) | 1.516 |
| F(0 0 0) | 2576 |
| Temperature (K) | 293(2) |
| Difractometr | Bruker P4 |
| Radiation | Mo-Kα (λ = 0.71073 Å) |
| 2θmax (°) | 56.22 |
| Range h, k, l | –23 ≤ h ≤ 24, –15 ≤ k ≤ 14, –35 ≤ l ≤ 17 |
| μ (mm−1) | 0.434 |
| Reflections measured | 12361 |
| Unique reflections | 9048 |
| Rint | 0.0579 |
| Observed (I > 2σ(I)) | 7002 |
| Refined parameters | 821 |
| Restraints | 2 |
| R1, wR2 (I > 2σ(I)) | R1 = 0.0685, wR2 = 0.1796 |
| R1, wR2 (all data) | R1 = 0.0932, wR2 = 0.1955 |
| Goodness-of-fit on F2 | 1.099 |
| (Δρ)max (e Å–3) | 0.498 |
| (Δρ)min (e Å–3) | –0.461 |
3 Results and discussion
The coordination polymer [Fe(4,4′-bpy)3(H2O)2](PF6)2·2(4,4′-bpy)·5 H2O (1) is prepared from FeSO4·7 H2O and 4,4′-bipyridine in a 1:5 molar ratio in water, followed by addition of KPF6. This directly gives single crystalline material of 1 in 86% yield. 1 could also be prepared from Fe(II)Fe(III)2 cluster [Fe3O(CH3COO)6(H2O)3], which obviously serves only as source of Fe(II). The effective magnetic moment of 4.84 B.M. (at 300 K) is characteristic for isolated high-spin Fe(II).
The crystallography study shows that iron(II) cations in the complex 1 are joined into infinite cationic chains by 4,4′-bipyridine bridges. The chains run along crystallographic b axis. Each iron atom has a distorted octahedral coordination environment and is coordinated additionally by two water molecules (mutually trans) and by two terminal 4,4′-bipyridine molecules (also mutually trans) (Fig. 1). Selected interatomic distances and angles are given in Table 2.
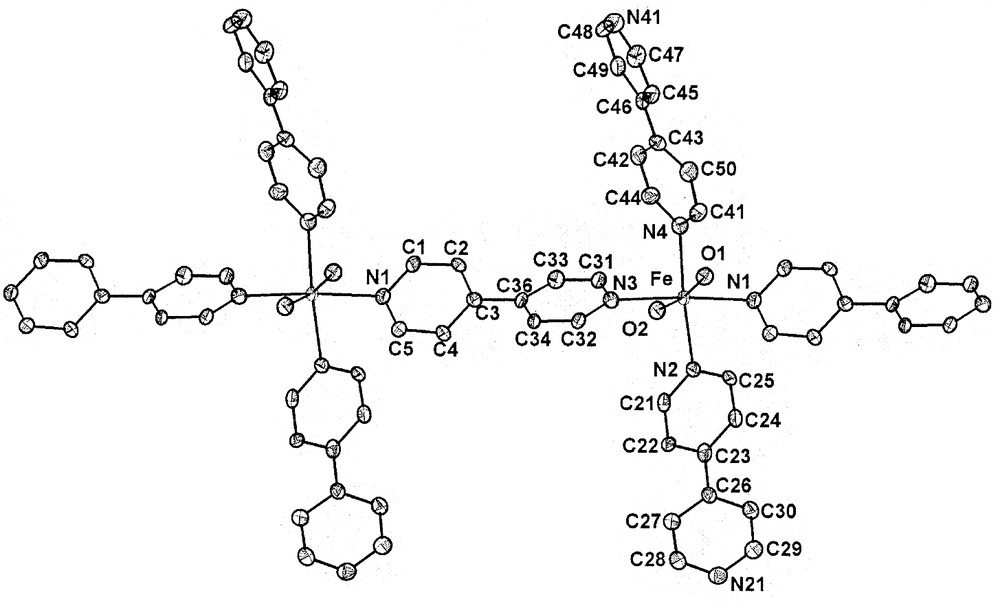
Fragment of infinite cationic chain [Fe(4,4′-bpy)3(H2O)2]n2n+ with the atom-labeling scheme and 50%-probability thermal ellipsoids. Hydrogen atoms are omitted for clarity.
Selected bond distances (Å) and angles (°) in 1
| Bond type | Distance (Å) | Angle | Value (°) | Angle | Value (°) |
| Fe–O1 | 2.096(5) | O1–Fe–O2 | 171.8(2) | O1–Fe–N4 | 88.1(2) |
| Fe–O2 | 2.086(6) | O1–Fe–N1 | 91.2(2) | O2–Fe–N4 | 85.0(2) |
| Fe–N1 | 2.222(5) | O2–Fe–N1 | 93.1(2) | N1–Fe–N2 | 86.2(2) |
| Fe–N2 | 2.235(7) | O1–Fe–N2 | 90.9(2) | N1–Fe–N3 | 176.2(3) |
| Fe–N3 | 2.295(5) | O2–Fe–N2 | 96.4(2) | N1–Fe–N4 | 89.5(2) |
| Fe–N4 | 2.239(7) | O1–Fe–N3 | 89.9(2) | N2–Fe–N3 | 90.1(2) |
| P–F | 1.594(4)–1.622(5)a | O2–Fe–N3 | 86.3(2) | N3–Fe–N4 | 94.2(2) |
a Data for not disordered anion.
Strictly linear chains are further packed up in such a way that non-bridging 4,4′-bipyridine ligands of neighboring chains are approximately parallel to each other with a dihedral angle 2.91° (Fig. 2). The ring planes are offset such that non-coordinated nitrogen atoms lie almost over the center of the rings containing nitrogen donor atom. The ring normal and the vector between the ring centroids form an angle of about 22°. The centroid–centroid distances of 3.9 Å between the planes of the 4,4′-bipyridine ligands indicate π–π stacking interactions between the adjacent 4,4′-bipyridine ligands [19]. Recently two related compounds, [M(4,4′-bpy)(4,4′-bpyEt)2(H2O)2](NO3)2·1.75(4,4′-bpyEt)·0.25(4,4′-bpy)·4.45 H2O (M = Zn, Cd), in which similar 1D chains, formed by metal cations, bridged by bipyridine molecules, are joined to 2D grids by stacking interactions and, in addition, by hydrogen bonds, have been reported [9]. In 1 the coordinated water molecules do not form hydrogen bonds to the non-coordinated nitrogen atoms of the bipyridine ligands in contrast to [M(4,4′-bpy)(4,4′-bpyEt)2(H2O)2](NO3)2·1.75(4,4′-bpyEt)·0.25(4,4′-bpy)·4.45 H2O. Thus these infinite chains form two-dimensional supramolecular layers only due to stacking interactions between slipped non-bridging 4,4′-bipyridine ligands of neighboring chains. Large rectangles of 11.666 × 13.825 Å size (taken as Fe…Fe distances) form in this way. In the resulting hydrophobic boxes reside anions [PF6]– (Fig. 2). A different type of packing of the linear …Fe(4,4′-bpy)–Fe(4,4′-bpy)… chains into layers is observed in [Fe(4,4′-bpy)(H2O)2(NCX)2]·(4,4′-bpy) (X = S, Se), where uncoordinated bpy connects adjacent linear chains via hydrogen bonds with coordinated water molecules into corrugated layers with somewhat larger Fe…Fe rectangles of 11.4 × 15.5 Å size [20].
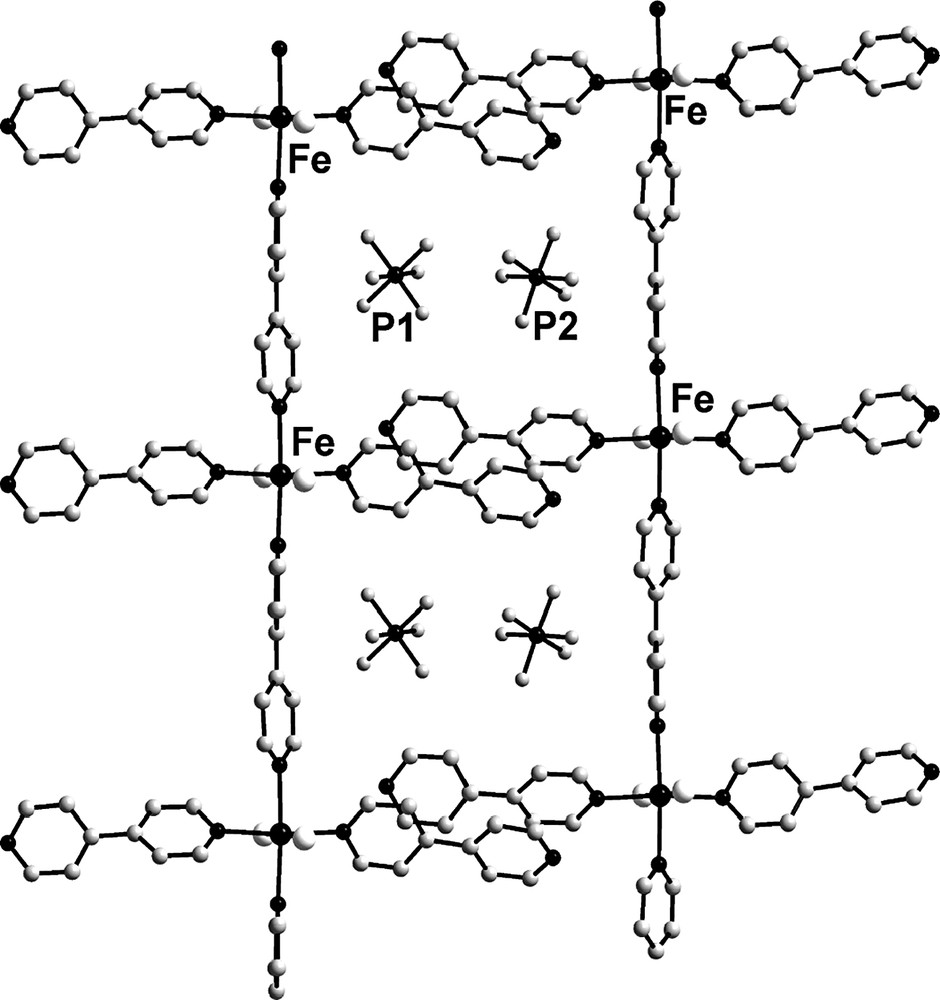
Packing of polymeric chains to the two-dimensional supramolecular layer (view along a axis). One of the anions (P2) was found to be disordered over two slightly different positions, only one orientation is shown. Hydrogen atoms, oxygen atoms of crystallization water and 4,4′-bipyridine guest molecules are omitted for clarity.
The supramolecular layers pack up in such a way, that the iron atoms of one layer lay exactly over iron atoms of another layer (Fe···Fe, 10.847(2) Å) and each cationic linear chain …Fe-(4,4′-bpy)–Fe-(4,4′-bpy)… is situated exactly above such chain of another layer. Two different types of clathrated 4,4′-bipyridine molecules (type A and B) are situated between the supramolecular layers. The coordinated water molecules form hydrogen bonds to the nitrogen atoms of type A clathrated 4,4′-bipyridine molecules (O1···N7 2.719(4), O2···N71 2.731 Å) (Fig. 3a). The H-bonded guest 4,4′-bipyridine molecules reside between the coordinated 4,4′-dipyridine ligands and exhibit intermolecular face-to-face π–π staking interaction with the centroid–centroid distances of 3.57–3.91 Å between the coordinated and clathrated 4,4′-bipyridine molecules. Type B 4,4′-bipyridine guest molecules are situated in T-shaped orientation to the coordinated ligands (Fig. 3b). This orientation corresponds to C–H...π interactions between the aromatic rings.

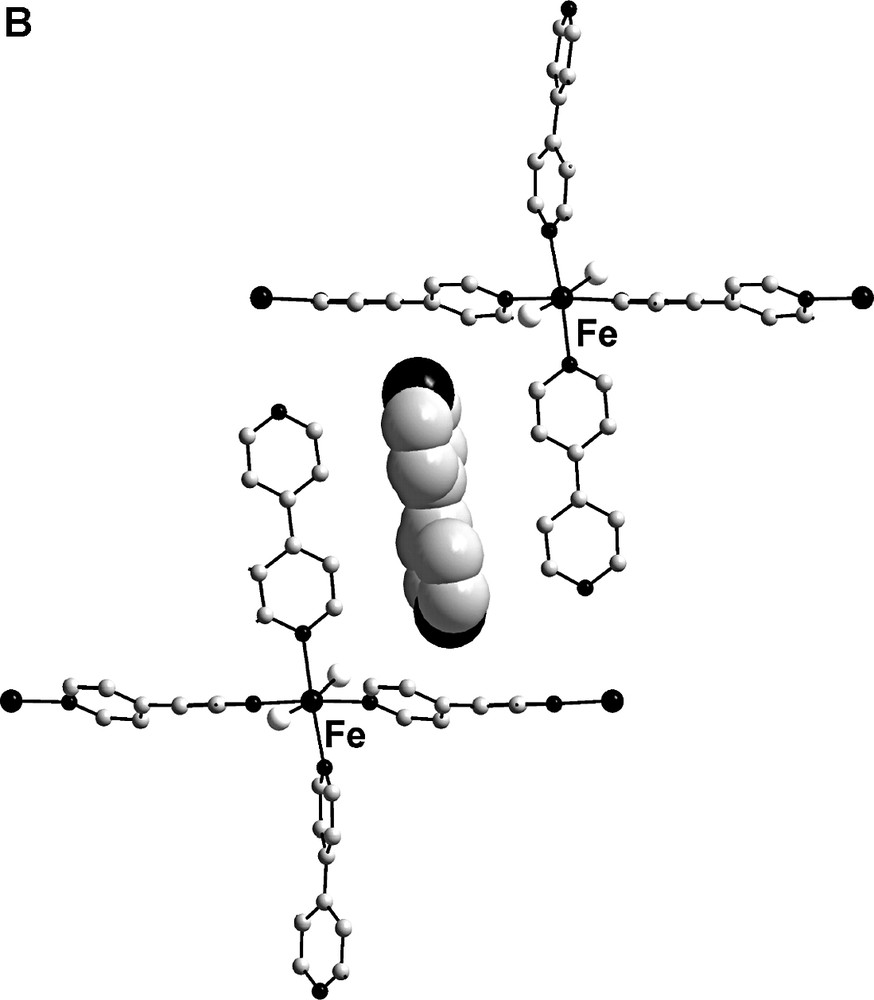
Clathration of 4,4′-bipyridine molecules: (a) face-to-face π–π staking and H-bonding; (b) C–H...π interactions. Hydrogen bonds are showen as dashed lines. 4,4′-bipyridine guest molecules are shown in Van-der-Waals radii. Hydrogen atoms are omitted.
Coordinated water molecules are directed to each other in such a way that hydrophilic channels are formed (Fig. 4). In these channels molecules of crystallization water are situated. They form hydrogen bonds with coordinated water molecules and between themselves (O···O, 2.597(13)–2.741(37) Å). The F4···O4W distance is 2.865(19) Å. It is thus possible to envision that the guest water molecule is connected to the anion through O–H···F hydrogen-bonding.
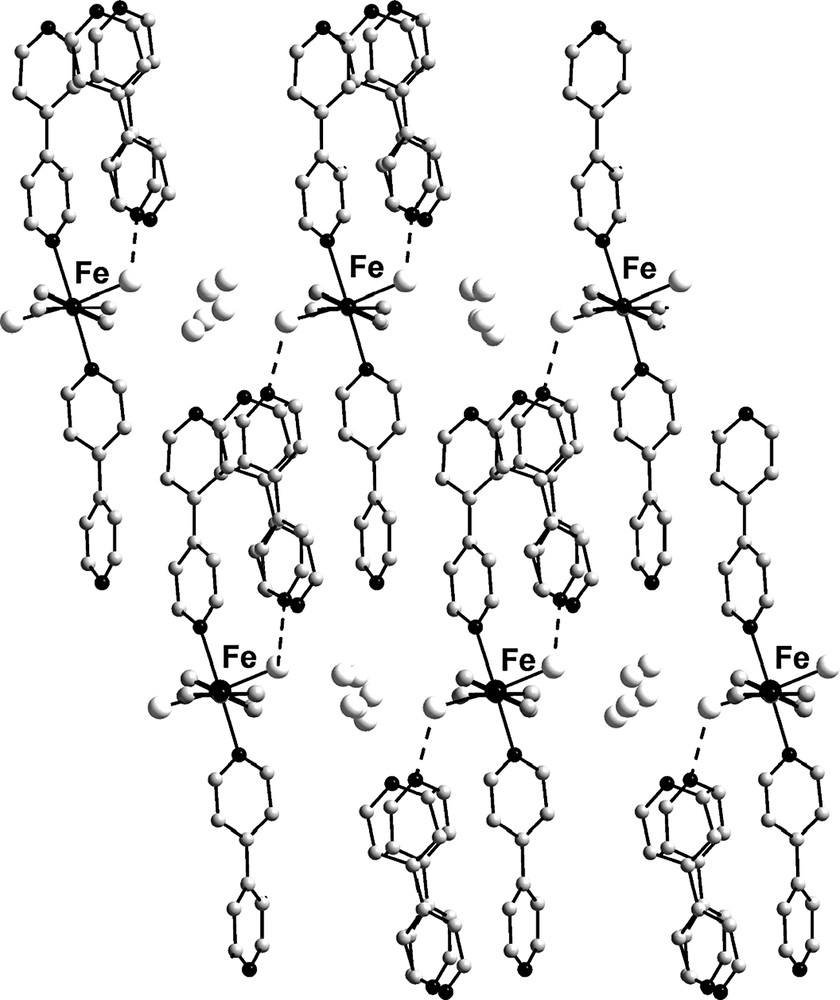
Packing of two-dimensional supramolecular layers in the crystal structure of 1 (view along b axis). Water molecules are shown as gray spheres. [PF6]– anions are omitted for clarity.
In summary, we have synthesized a new metal–organic coordination polymer [Fe(4,4′-bpy)3(H2O)2](PF6)2·2(4,4′-bpy)·5 H2O, in which cationic linear chains are joined by stacking interactions between non-bridging 4,4′-bipyridine ligands in supramolecular layers. The supramolecular layers form hydrophilic and hydrophobic channels filed by water and 4,4′-bipyridine guests, respectively. The structural similarity to the well-established family of coordination clathrates, [M(py)4X2] 2py is apparent and stems from the inability of metal center to accommodate more then four pyridine rings, and from the specific packing of these nearly flat aromatic ligands [21].
4 Supplementary materials
The supplementary material has been sent to the Cambridge Crystallographic Data Center, 12 Union Road, Cambridge, CB2 1EZ, UK as supplementary material No. 244410 (CIF) and can be obtained by contacting the CCDC (fax: +44-1223-336033; e-mail: deposit@ccdc.cam.ac.uk or www: http://www.ccdc.cam.ac.uk).
Acknowledgments
This work was supported by the Russian Foundation for Basic Research, grant no. 02-03-32604 and INTAS, grant no. 01-2346. NVI thanks Haldor Topsoe A/S for a fellowship. MNS is thankful to the Russian Science Support Foundation for a grant.


