1 Introduction
Octahedral metal clusters with six metal (M) atoms represented by the [M6X8] and [M6X12] type are well known and their structural and electronic properties have been investigated in detail [1,2]. The well-established compound W6Cl18 with an octahedral tungsten cluster of [W6Cli12]Cla6 is, however, considered as an exception of an [M6X12] type cluster because it hosts 18 electrons per cluster instead of the conventionally accepted maximum number of 16 electrons per cluster [3]. This number is even exceeded with the discovery of An[W6Cl18] compounds with A = (n-Bu)4N for n = 1 [4] and A = Li, K, Rb, Cs, Tl, Ag and Me4N, Et4N, NH4 for n = 2 [5,6].
Closely related with the composition of the binary W6Cl18 is the compound W6CCl18 that was recently discovered [7]. The crystal structure of W6CCl18 contains carbon-centered trigonal prismatic tungsten clusters. The structure of an individual W6CCl18 molecule is similar to a sulfur-centered triprismo-hexaniobium bromide fragment in A3[Nb6SBr17] compounds with A = K, Rb, Cs, Tl [8,9]. One striking difference of both crystal structures is that the [Nb6SBr16Br2/2]3– clusters are linked into chains by two shared Br atoms, and W6CCl18 forms a molecular structure. A3[Nb6SBr17] compounds have 14 electrons for Nb–Nb bonding, and according to electronic structure calculations there are only weak Nb–Nb interactions between adjacent cluster triangles in the prism.
The electrochemistry of W6CCl18 is remarkably rich. According to the cyclic voltammogram of (Bu4N)2[W6CCl18] five oxidation states should exist for [W6CCl18]n– with n = 0, 1, 2, 3, 4 [10]. Until now three [W6CCl18]n– containing compounds (n = 1, 2, 3) have been mentioned with Bu4N+ as counter cations. In addition, the gas phase dissociation of [W6CCl17]– anions was studied by mass spectroscopy as to investigate the formation mechanism of this unusual cluster compound [11].
Syntheses of W6CCl18 and the related W6CCl16 were performed by solid state reactions of WCl6 with carbons sources such as graphite, or CCl6 [7]. Other reactions of WCl6 with CCl4 have produced an X-ray amorphous precursor that can be extracted with HCl/(Bu4N)Cl to yield (Bu4N)2[W6CCl18] after recrystallization in CH3CN [10].
Recently we found a simple and efficient high yield synthesis for Lix[W6CCl18] that can open perspectives for the solution chemistry of carbon-centered triprismo-hexatungsten chlorides. With the synthesis and structure of the methanol solvated compound CsW6CCl18·CH3OH we here present the first example of this chemistry.
2 Syntheses
Cs[W6CCl18]·CH3OH was crystallized from a solution of Lix[W6CCl18] and CsCl in methanol. The Lix[W6CCl18] used in the reaction was synthesized by the solid state reaction of WCl4 with Li2CN2 in 2 to 1 molar ratio. The syntheses of WCl4, Li2CN2, and Lix[W6CCl18] are described as follows.
2.1 WCl4 [12]
WCl4 was synthesized by metallo-thermic reduction of WCl6 (Strem, 99.9%) with aluminium (shot, Strem, 99.999%). An evacuated silica tube charged with 9 mmol (3.569 g) WCl6 and 6.3 mmol (170 mg) Al was placed upright in a Simon–Müller furnace and heated at 370 °C for 48 h. After cooling to room temperature the WCl4 was found in the bottom part of the ampoule. To purify the product, one side of the ampoule was heated up to 270 °C, while the opposite side of the tube remained at room temperature so that volatile components such as the AlCl3 were sublimed to the colder part of the ampoule.
2.2 Li2CN2 [13]
Li2CN2 was prepared by reacting Li2CO3 (Merck, p. a.) with ammonia. In our reaction 12 mmol (0.9 g) of Li2CO3 were heated up to 610 °C in a continuous flow of ammonia. This temperature was held for 14 h. Afterwards the obtained reaction mixture was ground in an argon atmosphere and once more heated at 610 °C for 10 h in the ammonia flow. According to the X-ray powder diffraction pattern, the product contained only Li2CN2.
2.3 Lix[W6CCl18]
For the synthesis of Lix[W6CCl18], a mixture of WCl4 (0.5 mmol, 162.8 mg) and Li2CN2 (0.25 mmol, 13.5 mg) was ground in an argon atmosphere, sealed in an evacuated silica ampoule and then heated in a tube furnace at 500 °C for 12 h. After cooling to room temperature Lix[W6CCl18] was obtained as a black crystalline material that remains stable in air at least for about 1 week (as indicated by XRD). The reaction of WCl4 with Li2C2 results in the same but less crystalline product.
2.4 Cs[W6CCl18] · CH3OH
When Lix[W6CCl18] was dissolved in methanol the color of the solution turned dark green. Afterwards CsCl (Merck, 99.5%) was also dissolved in methanol and added to the solution of Lix[W6CCl18] in methanol. During the evaporation of the solvent, black crystals of Cs[W6CCl18]·CH3OH were obtained at the wall of an open beaker.
3 XRD and structure determination
All starting materials and products described in the syntheses section were checked by powder X-ray diffraction (XRD). The powder patterns were recorded on a StadiP diffractometer (STOE, Darmstadt), using germanium monochromated Cu Kα1 radiation (λ = 1.540598 Å) and a position sensitive X-ray detector (opening angle: 2θ = 6°). Routine analyses were done in the 2θ range between 10 and 60°. The powder patterns were indexed using Louer’s algorithm (DICVOL) [16,17].
Lix[W6CCl18] was characterized by X-ray powder diffraction without detectable side phases, except for the coproduced LiCl. The crystal structure of Lix[W6CCl18] was refined from a powder XRD pattern, without localizing the lithium positions (hexagonal, a = 88,648(9) Å, c = 17,490(1) Å). The lithium content in Lix[W6CCl18] could be x = 1 or higher. Some cluster compounds have shown capabilities to host variable Li contents because their metal states are flexible to varying electron counts. More detailed studies on the parent LiNb6Cl19 compound have revealed lithium contents corresponding to Li1+xNb6Cl19 with x = 0–4 [14,15].
Suitable crystals of Cs[W6CCl18] · CH3OH were selected and mounted on the tip of glass fibers for XRD studies. The measurements were performed on an IPDS (STOE) in the θ range between 2.74° and 27.91° at 205 K using graphite monochromated Mo Kα radiation (λ = 0.71073 Å). The intensity data were corrected by STOE software for Lorentz, polarization, and absorption effects. Out of the total 47 914 collected reflections, 6577 reflections were merged as unique. The structure was solved by direct methods (SHELXS-97) and refined by full-matrix least-squares calculations on F2 with merohedral twinning (SHELXL-97) [18]. The twin parts in the non-centrosymmetric space group P212121 were 45(1)% and 55(1)%, respectively. H atoms of the methanol were added using the H-FIX command. Anisotropic refinement of all atoms, except the H atoms, with fixed full occupancies yielded R1 = 0.0350 and wR2 = 0.0726 for all reflections. The largest residual peak and deepest hole in the fourier map were 2.6 e–Å–3 (84 pm from W) and –1.5 e–Å–3. Selected crystallographic data and measuring conditions are presented in Table 1. The atomic positions along with the isotropic displacement parameters are given in Table 2. Selected bond length and angles are shown in Table 3. Additional material can be ordered referring to the no. CSD 414367, names of the authors and citation of the paper at the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany.
Crystal data and structure refinement of Cs[W6CCl18] · CH3OH
| Formula | Cs[W6CCl18] · CH3OH |
| Crystal size | 0.22 × 0.07 × 0.05 mm3 |
| Crystal system | Orthorhombic |
| Space group; Z | P212121 (Nr. 19); 4 |
| Unit cell parameters | a = 9.6957(5) Å |
| b = 14.046(1) Å | |
| c = 20.238(1) Å | |
| Volume | 2.7561(3) nm3 |
| Formula weight | 1918.16 g/mol |
| Calculated density | 4.623 g/cm3 |
| Absorption coefficient | 27.988 mm–1 |
| F(000) | 3316 |
| Diffractometer, wavelength | STOE-IPDS, Mo–Kα (71.073 pm) |
| Temperature | 205(2) K |
| θ range | 2.74 ≤ θ ≤ 27.91° |
| Index ranges | –12 ≤ h ≤ 12, –18 ≤ k ≤ 18, –26 ≤ l ≤ 26 |
| Reflections collected | 47914 |
| Independent reflections | 6577 (Rint = 0.0755) |
| Completeness to θ = 27.91° | 99.6% |
| Absorption correction | Numerical, X-Red, X-Shape |
| Min., max. transmission | 0.0434, 0.4841 |
| Structure refinement | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6577/0/254 |
| Goodness-of-fit | 1.137 |
| Final R indices [I > 2σ(I)] | R1 = 0.0320, wR2 = 0.0718 |
| R indices (all data) | R1 = 0.0350, wR2 = 0.0726 |
| Largest difference peak | 2.569 e–/(pm3·106) (84 pm from W) |
| Absolute structure parameter | 0.55(1) |
Atomic coordinates and isotropic-equivalent displacement parameters (pm2·10–4) for Cs[W6CCl18]·CH3OH
| Atom | x | y | z | U(eq) c |
| Cs(1) | 0.7378(1) | –0.1168(1) | 0.0121(1) | 0.0418(2) |
| C(1) | 0.7392(7) | 0.2568(7) | 0.1831(4) | 0.009(2) |
| W(1) | 0.9012(1) | 0.1581(1) | 0.1562(1) | 0.0163(1) |
| W(2) | 0.9117(1) | 0.3478(1) | 0.1546(1) | 0.0159(1) |
| W(3) | 0.8748(1) | 0.2559(1) | 0.2689(1) | 0.0170(1) |
| W(4) | 0.5851(1) | 0.1652(1) | 0.1377(1) | 0.0149(1) |
| W(5) | 0.5948(1) | 0.3552(1) | 0.1349(1) | 0.0149(1) |
| W(6) | 0.5590(1) | 0.2639(1) | 0.2492(1) | 0.0167(1) |
| Cl(1)a | 0.0236(3) | 0.0237(2) | 0.1122(2) | 0.0312(7) |
| Cl(2)a | 0.0518(3) | 0.4745(2) | 0.1097(2) | 0.0314(7) |
| Cl(3)a | 0.9618(3) | 0.2547(3) | 0.3802(1) | 0.0302(6) |
| Cl(4)a | 0.4762(3) | 0.0348(2) | 0.0810(2) | 0.0267(7) |
| Cl(5)a | 0.4985(3) | 0.4871(2) | 0.0749(2) | 0.0291(7) |
| Cl(6)a | 0.4122(3) | 0.2699(3) | 0.3453(2) | 0.0339(7) |
| Cl(7)i,b | 0.0894(2) | 0.2475(2) | 0.1107(1) | 0.0245(5) |
| Cl(8)i,b | 0.0575(3) | 0.3665(2) | 0.2493(2) | 0.0261(6) |
| Cl(9)i,b | 0.0444(3) | 0.1345(2) | 0.2516(2) | 0.0269(6) |
| Cl(10)i,p | 0.7267(3) | 0.0392(2) | 0.1875(2) | 0.0208(6) |
| Cl(11)i,p | 0.7652(3) | 0.1467(2) | 0.0533(2) | 0.0193(5) |
| Cl(12)i,p | 0.7779(3) | 0.3619(2) | 0.0514(2) | 0.0190(5) |
| Cl(13)i,p | 0.7481(3) | 0.4759(2) | 0.1822(2) | 0.0206(6) |
| Cl(14)i,p | 0.7078(3) | 0.3737(2) | 0.3113(2) | 0.0221(6) |
| Cl(15)i,p | 0.6959(3) | 0.1489(2) | 0.3142(2) | 0.0229(6) |
| Cl(16)i,b | 0.4385(2) | 0.2615(2) | 0.0704(1) | 0.0230(5) |
| Cl(17)i,b | 0.4047(3) | 0.3825(2) | 0.2070(2) | 0.0256(6) |
| Cl(18)i,b | 0.3931(3) | 0.1493(2) | 0.2111(2) | 0.0242(6) |
| O(1) | 0.878(1) | –0.3098(8) | –0.0207(5) | 0.040(2) |
| C(2) | 0.785(2) | –0.370(1) | 0.009(1) | 0.047(4) |
| H(11) | 0.9411 | –0.2983 | 0.0004 | 0.0219 |
| H(21) | 0.8141 | –0.4354 | 0.0030 | 0.0564 |
| H(22) | 0.6946 | –0.3613 | –0.0111 | 0.0564 |
| H(23) | 0.7790 | –0.3556 | 0.0558 | 0.0564 |
c U(eq) is defined as one third of the trace of the orthogonalized Uij tensor.
Selected bond lengths (Å) and angle (°) in Cs[W6CCl18]·CH3OH
| W(1)–W(2) | 2.6658(6) | W(2)–Cl(8) i,b | 2.396(3) |
| W(1)–W(3) | 2.6749(7) | W(3)–Cl(8) i,b | 2.390(3) |
| W(2)–W(3) | 2.6735(6) | W(3)–Cl(9) i,b | 2.394(3) |
| W(1)–W(4) | 3.0897(6) | W(1)–Cl(9) i,b | 2.400(3) |
| W(2)–W(5) | 3.1000(6) | W(1)–Cl(10) i,p | 2.461(3) |
| W(3)–W(6) | 3.0898(5) | W(4)–Cl(10) i,p | 2.456(3) |
| C(1)–W(1) | 2.165(9) | W(1)–Cl(11) i,p | 2.471(3) |
| C(1)–W(4) | 2.176(8) | W(4)–Cl(11) i,p | 2.457(3) |
| C(1)–W(3) | 2.178(8) | W(2)–Cl(12) i,p | 2.468(3) |
| C(1)–W(2) | 2.183(9) | W(5)–Cl(12) i,p | 2.453(3) |
| C(1)–W(5) | 2.196(9) | W(2)–Cl(13) i,p | 2.463(3) |
| C(1)–W(6) | 2.202(8) | W(5)–Cl(13) i,p | 2.450(3) |
| W(1)–Cl(1) a | 2.401(3) | W(3)–Cl(14) i,p | 2.469(3) |
| W(2)–Cl(2) a | 2.416(3) | W(6)–Cl(14) i,p | 2.458(3) |
| W(3)–Cl(3) a | 2.405(3) | W(3)–Cl(15) i,p | 2.470(3) |
| W(4)–Cl(4) a | 2.405(3) | W(6)–Cl(15) i,p | 2.470(3) |
| W(5)–Cl(5) a | 2.404(3) | ||
| W(6)–Cl(6) a | 2.413(3) | W(2)–W(1)–W(3) | 60.08(2) |
4 Results and discussion
W6CCl18 was reported as the first tungsten compound containing a carbon-centered trigonal prismatic [W6C] cluster [7]. Until now a high-yield synthesis for this compound was not available. During our reaction studies of tungsten halides with Li2CN2 or Li2C2 we obtained the lithium salt Lix[W6CCl18], containing a similar, but anionic cluster compared to W6CCl18. This compound has served as a starting material for the synthesis of Cs[W6CCl18]·CH3OH through an ion exchange reaction in methanolic solution. Both compounds represent the first examples of alkali metal compounds with this cluster anion. The synthesis and structure of Ca[W6CCl18] will be reported in a different contribution [19]. The discovery of more An[W6CCl18] compounds where A may denote a group 1, 2, or another metal is an upcoming issue.
The crystal structure of Cs[W6CCl18].CH3OH contains [W6C] cluster cores with a trigonal prismatic arrangement of tungsten atoms, centered by a carbon atom. The W–W distances along the triangular edges of the prism (2.6658(6)–2.6749(7) Å) are only slightly longer than the sum of the covalent radii of two tungsten atoms (2.60 Å [20]) yielding a bond order of about 0.8. The edges of the prism, representing the connections between adjacent triangles of tungsten atoms, are clearly longer (3.0897(6)–3.1000(6) Å) than the bond distances within the triangles, and yield a bond order of only about 0.15. According to this estimation we may consider only weakly bonding W–W interactions along edges of the prism as already noted for [Nb6SBr18]2–. It may be, therefore, assumed that covalent W–C bondings are mainly responsible for the cohesion between the two trigonal tungsten clusters in each [W6C] unit. Using a tolerance of 3σ, all W–C distances are equal ((W–C) = 2.183(9) Å) and in accordance with the W–C distance (2.197 Å) in the structure of binary WC. However, the [W6C] blocks in the structure of WC exhibit nearly equivalent W–W distances (2.907 and 2.837 Å) [21].
The [W6C] building block in [W6CCl18] is surrounded by 12 edge bridging (inner, Cli) and 6 terminal (Cla) chlorine atoms, as shown in Fig. 1. The inner chlorine atoms can be further decomposed into six triangular basal edge bridging (Cli,b) and six prismatic edge bridging (Cli,p) chlorine ligands.

The [W6CCl18]– cluster unit in Cs[W6CCl18]·CH3OH.
If the [W6CCl18]– clusters of Cs[W6CCl18]·CH3OH are considered as spheres, their arrangement follows the motif of a hexagonal closest packing (hcp), with the stacking direction of hexagonal layers along [100] (Fig. 2). The stacking sequence in the structure is emphasized in Fig. 3, where only the centering carbon spheres of clusters are used to relate to a hcp of clusters. This packing, is however, relatively open to allow cesium ions and methanol molecules to occupy voids along the [100] direction in an alternating sequence (Fig. 2). Both of them reside almost in the triangular faces of each hexagonal layer rather than in octahedral interstices of a hcp (Fig. 3). The cesium ions are located in a 12-fold environment formed by 10 chlorine and two oxygen atoms (of CH3OH), but the coordination polyhedron is not regular as shown in Fig. 4. The Cs–Cl distances range from 3579(3) Å to 4172(3) Å, and the Cs–O distances are 3.11(1) Å and 3.64(1) Å. The average values of these distances can be related with the sum of the ionic radii of Cs (1.88 Å, C.N. = 12), Cl (1.81 Å), and O (1.40 Å), respectively, provided by Shannon [22].
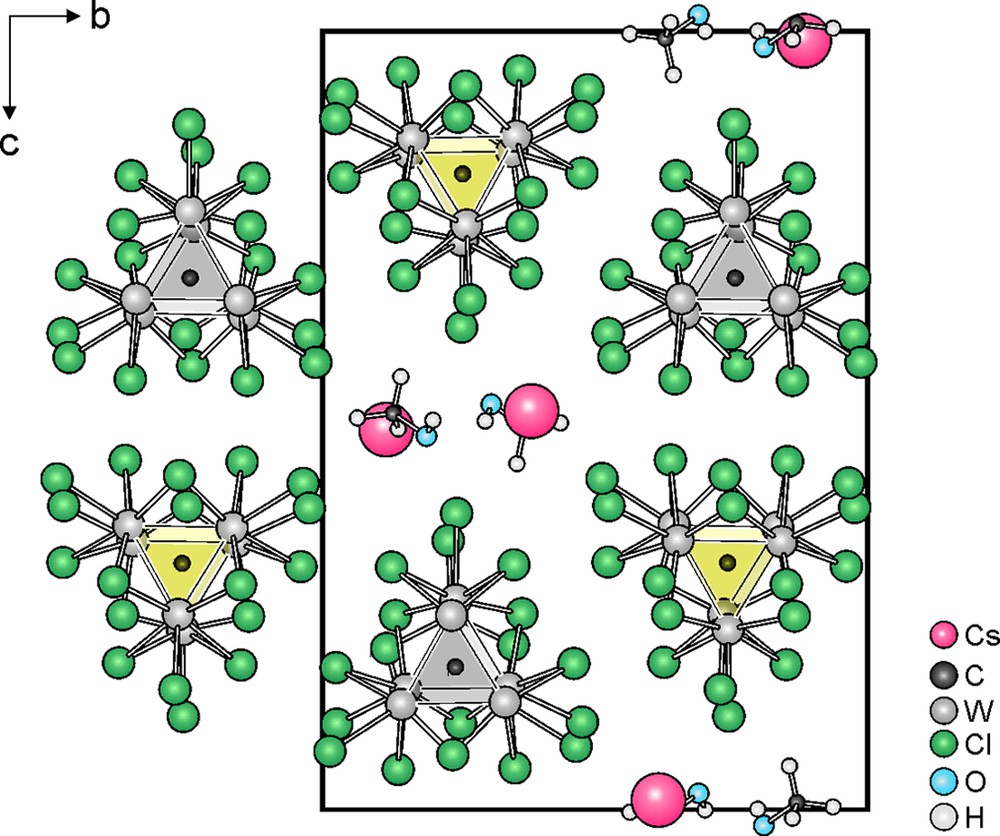
Projection of the Cs[W6CCl18]·CH3OH structure along [100].
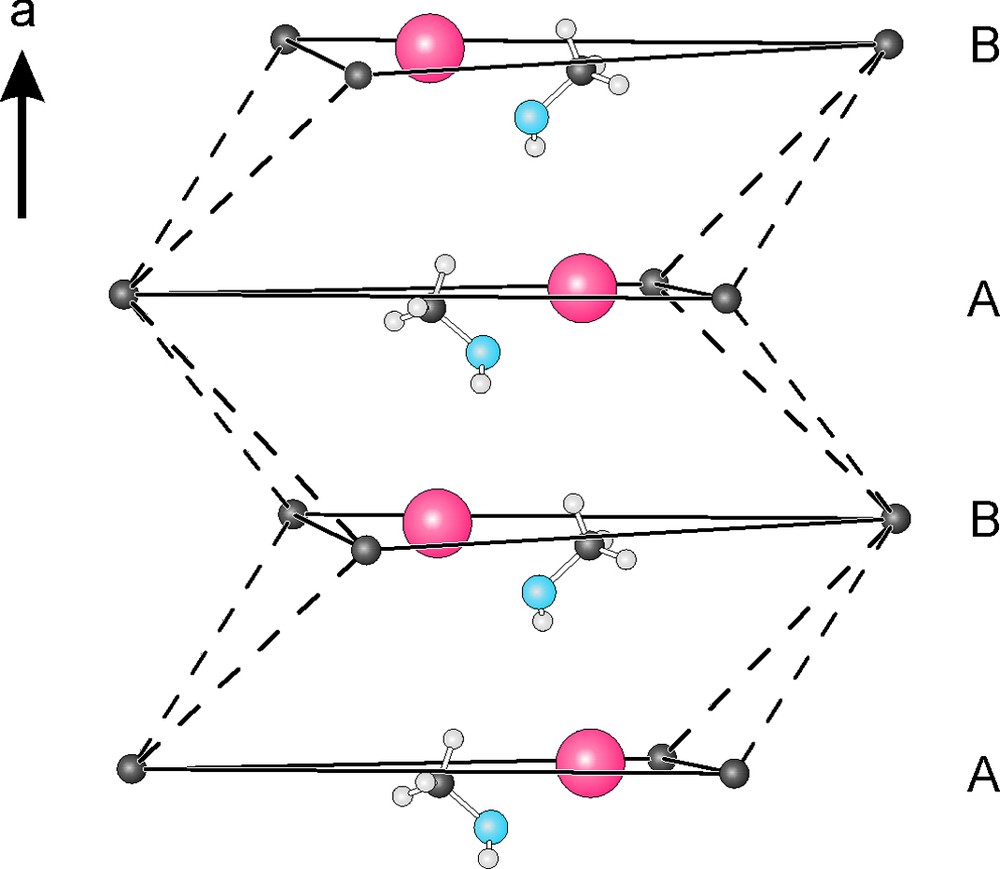
Sequence of hexagonal cluster layers (A, B, A…) with the cluster centers represented by their carbon atoms (shown in black). Cesium ions (red) and methanol molecules occupy positions within each hexagonal layer.

Coordination environment around the cesium ion.
[W6CCl18]n– ions ought to provide a multifaceted solution chemistry, leading to new compounds a) with different counter cations, b) with other interstitial atoms than carbon, and c) other ligands than chlorine. All this chemistry may be combined with different oxidation states of the clusters. To date, Lix[W6CCl18] may be regarded as a useful starting material for some of this chemistry.


