1 Introduction
The design of highly efficient photocatalytic systems, which can work efficiently for the purification of polluted water, is of vital interest in the research of environment friendly catalyst. The photocatalytic degradation of organic toxic compounds dissolved in aqueous solutions at low concentrations using irradiated small TiO2 particles is one of the most promising applications of photocatalysts [1–5]. It is attractive to use the hybrid materials, combined with adsorbents and photocatalysts for the purification of water [6,7]. Zeolite and mesoporous silica, which have high surface area and porous structure, have been used as conventional adsorbents and useful supports of photocatalyst. The condensation properties of these adsorbents should depend on their surface hydrophilic–hydrophobic properties and the highly hydrophobic surface seems to be suitable for the adsorption of organic compounds diluted in water [8]. In most cases, the surface of zeolites especially of mesoporous silica show hydrophilic properties, which are not suitable for the absorption of organic compounds diluted in water. The advanced modification of surfaces to become hydrophobic has been eagerly desired. The fine TiO2 photocatalyst loaded on hydrophobic zeolite and mesoporous silica have opened new possibilities for photocatalytic degradation of various organic compounds diluted water.
In the present study, therefore, TiO2 photocatalysts loaded on the fluoride-modified hydrophobic mesoporous silica were prepared using the combined utilization of tetraethylammonium fluoride (TEAF) as the source of the fluoride and dodecylamine (DDA) as templates. Using these hydrophobic mesoporous supports and TiO2 photocatalysts, the adsorption properties and photocatalytic reactivities for the degradation of various alcohols (2-propanol, 2-hexanol) diluted in water have been studied.
2 Experimental section
2.1 Preparation of photocatalysts
The synthesis of the hydrophobic mesoporous silica (denoted as HMS(F)) was performed using tetraethyl orthosilicate (TEOS), tetraethylammonium fluoride (TEAF) as the source of the fluoride and dodecylamine (DDA) as the template. TEOS dissolved in a mixture of 2-propanol and ethanol and DDA dissolved in water with HCl are mixed, following to stirring at 295 K for 24 h. The precursor mixture was washed by distilled water, dried at 373 K for 24 h, and then calcined at 823 K for 7 h. The ratio of TEAF to DDA was 0 (HMS), 0.25 (HMS(F1)), 0.75 (HMS(F3)) and 1.25 (HMS(F5)). Furthermore, impTi/HMS(F) (10 wt.% as TiO2) was prepared by impregnating HMS(F) with an aqueous titanium oxalate solution, then dried and calcined for 5 h at 773 K.
2.2 Photocatalytic reactions
The photocatalytic reactions were carried out with the catalysts (50 mg) in the quartz tube with an alcohol solution (2-propanol, 2-hexanol) (2.6 × 10−3 M, 25 ml). The sample was irradiated at 295 K using UV light (λ > 280 nm) from a 100-W high-pressure Hg lamp with stirring under O2 atmosphere in the system. The reaction products were analyzed by gas chromatography. The photocatalytic reactivity was estimated from the initial decrease in the concentration of alcohol after preadsorption of alcohol on the catalyst under dark condition. The adsorption isotherms measurement was carried out stirring the catalysts (10 mg) dissolved in the solutions (5 ml) without irradiation at 295 K.
2.3 Characterizations
The XRD patterns were recorded with a RIGAKU RINT 2500 using Cu Kα radiation. The diffuse reflectance absorption spectra were recorded with a Shimadzu UV-2400A photospectrometer. The XAFS spectra (XANES and EXAFS) were measured at the BL-9A facility of the Photon Factory at the National Laboratory for High-Energy Physics, Tsukuba. A Si(111) double crystal was used to monochromatize the X-rays from the 2.5-GeV electron storage ring. The Ti K-edge absorption spectra were recorded in the fluorescence mode at 295 K. The normalized spectra were obtained by a procedure described in previous literature [9] and Fourier transformation was performed on k3-weighted EXAFS oscillations in the range of 3–10 Å−1. The hydrophobicity of the catalysts was studied measuring the H2O adsorption isotherms of the catalysts. The reaction products were analyzed using g.c.
3 Results and discussion
Fig. 1 shows the reaction time profiles of the photocatalytic degradation of 2-propanol diluted in water on the impTi/HMS(F5). The photocatalytic reaction proceeds with the UV-irradiation. The concentration of 2-propanol decreased and acetone increased as the intermediate, finally 2-propanol and acetone were degradated into CO2 and H2O. Although the similar photocatalytic degradation proceeded also on other photocatalysts (impTi/HMS(F1~F3)), the adsorption properties and degradation performance depended on the types of supports.

The reaction time profiles of the photocatalytic degradation of 2-PrOH diluted in water on the impTi/HMS(F5).
Fig. 2 shows the adsorption isotherm of H2O molecules at 298 K obtained over various catalysts. The amount of adsorbed H2O molecules decreases with increasing the content of fluoride in the photocatalysts. The values of H2O adsorption ability per unit surface area of catalysts at P/P0 = 1 are 7.1 mmol m–2-cat. (HMS), 2.6 (HMS(F1)), 1.9 (HMS(F3)) and 1.5 (HMS(F5)). This shows that fluoride-modified mesoporous silica can be hydrophobilized by the fluoride-modifications.
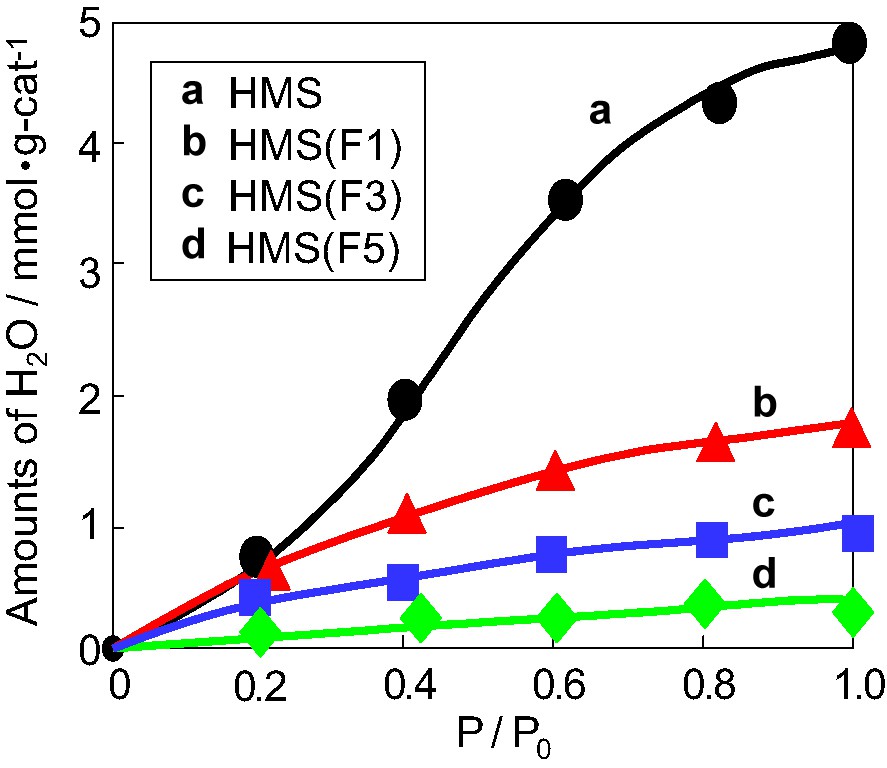
The adsorption isotherms of H2O at 298 K with (a) HMS, (b) HMS(F1), (c) HMS(F3), (d) HMS(F5).
Fig. 3 shows the effect of the fluorination of impTi/HMS on the photocatalytic degradation of 2-propanol and 2-hexanol diluted in water. It is known that the hydrophobicity of hexanol is higher than that of 2-propanol. Those results indicate that the more treatment of fluoride-modified for mesoporous silica, the more efficiently photocatalytic reactivities can be observed. This enhancement of the reactivity by the fluoride modification can be observed more clearly with the more highly hydrophobic reactant (hexanol).
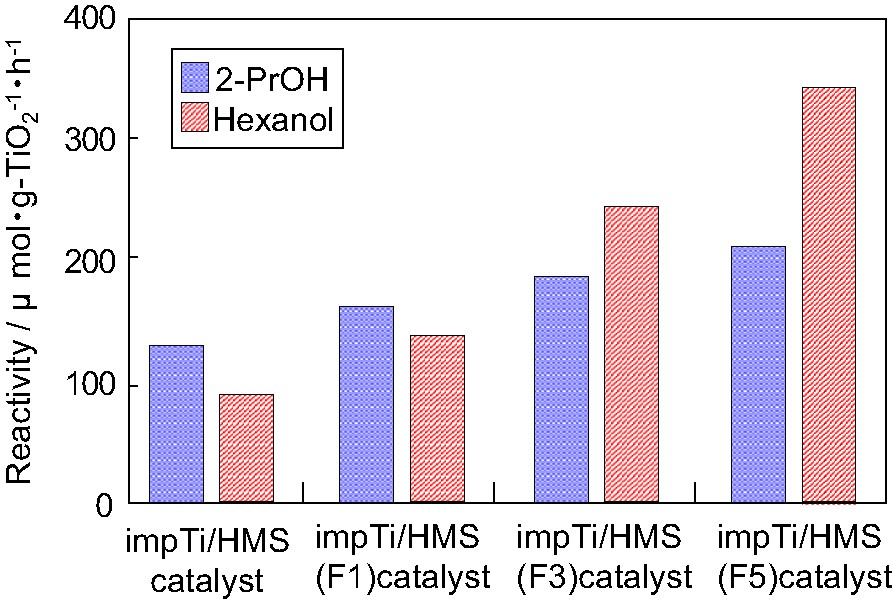
Effect of the fluorination of impTi/HMS on the photocatalytic degradation.
To account for the enhancement in the photocatalytic reactivities of the impTi/HMS(F) catalysts as compared to the non-modified impTi/HMS, the adsorption properties of 2-propanol and 2-hexanol on these catalysts were studied. Fig. 4 shows the effect of the fluorination of impTi/HMS on the adsorption of alcohols. The more treatment of fluoride-modified for mesoporous silica, the more amount of adsorption with hydrophobic reactant (hexanol) can be observed. The amount of adsorbed reactant increased with increasing the amount of fluoride species, indicating that highly selective adsorption of organic compounds in their aqueous solutions can be realized by hydrophobilizing the support. Furthermore, it can be found that the more highly adsorption, the more efficiently photocatalytic reactivities were promoted.

Effect of the fluorination of impTi/HMS on the adsorption of alcohols.
Fig. 5 shows UV–Vis adsorption spectra of impTi/HMS(F). In the UV–Vis spectra, the impTi/HMS catalysts exhibited the absorption band at around 260–360 nm, indicating the presence of the fine particle of anatase TiO2. The edge of adsorption of TiO2 shifted to longer wavelength regions with increasing the content of fluoride in the mesoporous silica. Because the smaller particle of TiO2 crystalline usually exhibits the absorption band at the shorter wavelength regions, the present shift to the longer wavelength regions suggests that the crystallinity of fine anatase TiO2 increased. This shows that the crystallization of TiO2 is increased on the mesoporous silica support with the higher content of fluoride.
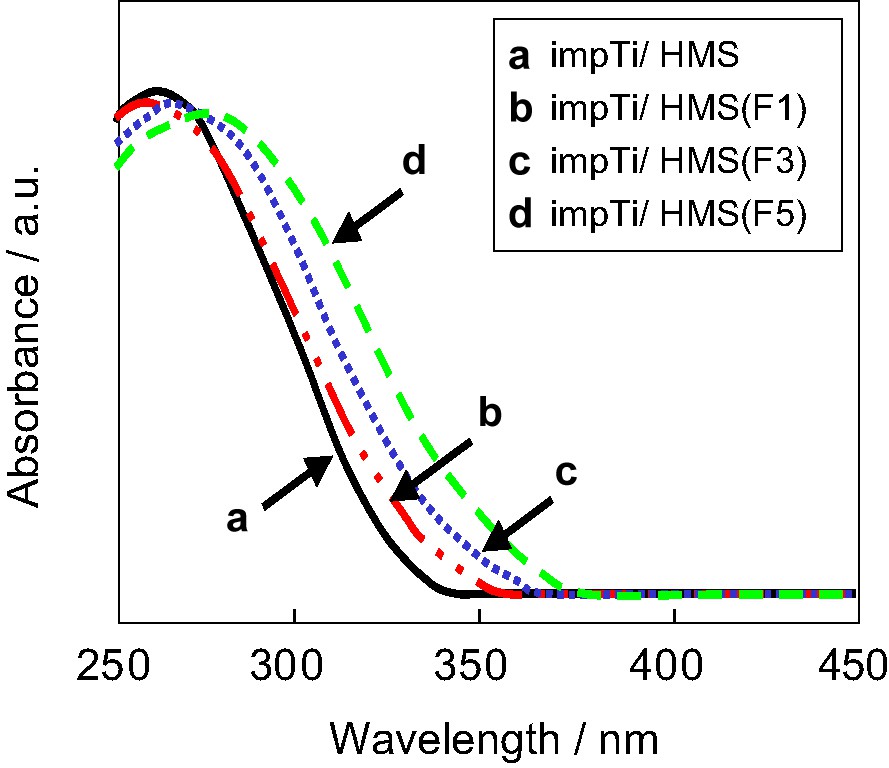
UV–Vis adsorption spectra of impTi/HMS with (a) impTi/HMS, (b) impTi/HMS(F1), (c) impTi/HMS(F3), (d) impTi/HMS(F5).
Fig. 6 shows XRD patterns of impTi/HMS. The XRD patterns of the prepared silica catalysts (HMS(F)) exhibited well-resolved peak typical of hexagonal structure of the HMS mesoporous molecular sieves having pores larger than 20 Å [10,11]. The peak intensity of anatase TiO2 is very weak but increases with increasing the content of fluoride in the mesoporous silica. This shows that the crystallization of TiO2 increased with increasing the content of fluoride in the mesoporous silica as already estimated from the results obtained in the UV–Vis measurement (Fig. 5).

XRD patterns of impTi/HMS with (a) impTi/HMS, (b) impTi/HMS(F1), (c) impTi/HMS(F3), (d) impTi/HMS(F5).
Fig. 7 shows the XAFS (XANES and FT-EXAFS) spectra of the impTi/HMS catalysts. The XANES spectra of the Ti containing compounds at the Ti K-edge show several well-defined preedge peaks that are related to the local structures surrounding the Ti atom. These relative intensities of the preedge peaks provide useful information on the coordination number surrounding the Ti atom [12]. The XANES spectra of these catalysts at the Ti K-edge exhibit the preedge peak branched off into three distinct weak peaks. The FT-EXAFS spectra exhibit the existence of the peaks attributed to the neighboring O atoms (Ti–O) and the neighboring Ti atoms (Ti–O–Ti). These XAFS (XANES and FT-EXAFS) results indicate that the TiO2 loaded on the mesoporous silica exists in the mixture of anatase and rutile phases. From the change in the relative intensities of preedge peaks in the XANES spectra and the change in the intensities of bands in FT-EXAFS spectra (Ti–O–Ti), it is easily estimated that the fine particles of anatase TiO2 crystalline existed as main component. In combination with the results estimated from the UV–Vis absorption and XRD patterns shown in Figs. 5 and 6, it can be concluded that the well-crystallized anatase TiO2 fine particles can be formed easily on the fluoride-modified surface. This fine anatase TiO2 can be responsible for the efficient photocatalytic reactivity.

XANES (A–D) and FT-EXAFS (a–d) spectra of the imp Ti/HMS(F). (A,a) impTi/HMS, (B,b) impTi/HMS(F1), (C,c)impTi/HMS(F3), (D,d) impTi/HMS(F5).
4 Conclusions
The impTi/HMS(F) exhibited the higher photocatalytic reactivity than impTi/HMS. The larger enhancement of the reactivity can be observed on catalysts with increasing the content of fluoride in the mesoporous silica. The effects of fluoride-modified HMS with increase in the amount of added fluoride are follows: (1) the amount of H2O adsorption was decreased, (2) the amount of adsorbed organic reactant was increased, and (3) the well-crystallized anatase TiO2 was easily formed. By these reasons, impTi/HMS(F) exhibited the higher reactivity for photocatalytic degradation in liquid phase, because the hydrophobic mesoporous surface is suitable to condensed the organic compounds and can transfer the reactants to the well-crystallized anatase TiO2 fine particle photocatalysts formed in the mesopores.
Acknowledgements
This work is partly performed under the project of collaborative research at the Joining and Welding Research Institute (JWRI) of Osaka University. The X-ray adsorption experiments were performed at the Photon Factory of KEK (20003G251).


