1 Introduction
Both Mo and Re form numerous cuboidal clusters with chalcogenide bridges of general type M4(μ3-Q)4n+ (M = Mo, n = 4–6; M = Re, n = 8; Q = S–Te). The Mo clusters display rich electrochemistry, and in recent years the cyanide derivatives, [M4Q4(CN)12]n– (M = Mo, n = 6; M = Re, n = 4) were widely used for construction of coordination polymers via cyanide bridges [1–8]. There are some indications that mixed Mo/Re cuboidal clusters of this type can exist. Thus a series of isostructural solids MoxRe4–xS4Te4 (x = 0, 0.67, 1.0, 1.33, 2) was prepared, and with the increase in x a transition from Re4S48+ to MoRe3S48+ and Mo2Re2S48+ as chief building blocks in these clusters was postulated [9]. The reduced tetrahedra Mo2Re2S4n+ (n = 6, 7) are also found in MMo2Re2S8 [10]. A Mo3ReS4n+ (n most likely 5 or 6) cluster was reported but serious crystallographic problems did not allow the unambiguous charge assignment in its NCS– derivative [11]. A hydride-bridged carbonyl cluster, [Mo3Re(μ3-H)4(CO)12]3–, was prepared by a cluster self-assembly from [ReH9]2– and [Mo(CO)6] or [Mo(CO)3(diglyme)] [12]. In this note we report the preparation of a Mo3ReS46+ cluster, [Mo3ReS4(dtp)6], and its oxidation product with an unusual, highly oxidized Mo3ReS49+ core, [Mo3ReS4(O)2(dtp)5] (dtp = (EtO)2PS2).
2 Experimental
2.1 Preparation
Five milliliters of a 42-mM solution of [Mo3S4(H2O)9]4+ [13] in 4 M HCl were heated with 85 mg (0.21 mmol) of [Re(CO)5Br] (Aldrich) in a Teflon-lined high-pressure reactor (140 °C, 48 h) under nitrogen. The resulting brown solution was filtered, diluted to [H+] = 0.3 M and loaded on a Dowex 50-WX2 cation-exchanger. After washing with 100 ml of 1 M HCl to remove unreacted [Mo3S4(H2O)9]4+ (green band), the red-brown band was eluted with 2.5 M HCl. To this solution 0.5 g of solid Kdtp (made by dissolving P4S10 in EtOH and neutralizing with KOH) was added. The bluish-brown precipitate, consisting mainly of [Mo3ReS4(dtp)6] (1), was collected, washed with water and methanol, dissolved in acetone followed by addition of an equal volume of methanol and was left in an open vial to crystallize. After 4 days a crop of blue single crystals of [Mo3ReS4(O)2(dtp)5] (2) of X-ray quality was obtained (yield = 55%). Anal. Calc. (found) S, 28.76 (27.84); C 15.40 (15.35); H 3.23 (3.28) %.
2.2 X-ray crystallography
The data collection were performed on a Bruker Smart CCD diffractometer using graphite-monochromated Mo Kα radiation (λ = 0.71073 Å). A hemisphere of data were collected based on three ω-scan runs (starting ω = –28°) at values ϕ = 0°, 90° and 180° with the detector at 2θ = 28°. At each of these runs, frames (606, 435 and 230, respectively) were collected at 0.3° intervals and 25 s per frame. The diffraction frames were integrated using the SAINT package and corrected for absorption with SADABS [14,15]. The positions of the heavy atoms were determined by direct methods and successive difference electron density maps using the SHELXTL 5.10 software package were calculated to locate the remaining atoms [16]. The refinement was performed by the full-matrix-least square method based on F2. All cluster atoms except the ethoxy fragments on the dithiophosphate ligands were refined anisotropically. Two metallic atoms were found in the asymmetric unit, namely Mo(1)/Re(1) and Mo(2), both in general position being the former atomic position partially occupied (1:1) by one molybdenum and one rhenium atom. At this moment the highest peak of the Fourier map, which lies on a general position, was assigned to one oxygen atom O (100) from water molecules of crystallization and refined isotropically with half occupancy. In this model, O (100) appears at 1.21 (7) Å from the symmetry related O (100B) atom (generated by the operation B: –x + 1/2, –y + 1/2, –z + 2), this short distance has no chemical meaning and is merely due to crystallographic disorder also reflected in the high thermal parameters of these oxygen atoms. The positions of all hydrogen belonging to the ethyl fragments were generated geometrically, assigned isotropic thermal parameters and allowed to ride on their respective parent carbon atoms while hydrogen atoms in water molecules were not included in the refinement.
Crystal data for [2]: Mo3S14ReP5O13C20H52, M = 1578.33, monoclinic, space group C2/c, a = 17.244 (6), b = 18.838 (7), c = 17.048 (6), β = 101.281 (10) V = 5431 (3) Å3, T = 293 K, Z = 4, ρcalcd = 1.930 g cm–3, μ (Mo Kα) = 3.630 mm–1. Crystal size: 0.18 × 0.16 × 0.14 mm3. Reflections collected/unique = 18766/6247 (Rint = 0.0867). Final refinement converged with R1 = 0.0730 for 3164 reflections with F0 > 4σ (F0) and wR2 = 0.2270 for all reflections, GoF = 1.030, max/min residual electron density 1.39/–1.14 e Å–3. Crystallographic data (excluding structure factors) for the structure reported in this paper have been deposited with the Cambridge Crystallographic Data Center as supplementary publication no. CCDC 249357. Copies of data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html or from the Cambridge Crystallographic Data Center, 12, Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336 033; E-mail: deposit@ccdc.cam.ac.uk.
3 Results and discussion
A [3 + 1] cluster assembly by addition of formally Re+ (as [Re(CO)5Br]) to the Mo3S44+ core selectively gives a Mo3ReS4 cluster, which probably exists in HCl solutions as [Mo3ReS4(H2O)12]n+ (n = 5 or 6), or even as [Mo3((Re(CO)3)S4(H2O)9]5+. Ligand substitution takes place upon addition of dithiophosphate, leading first to the formation of a bluish-brown [Mo3ReS4(dtp)6] (1) complex. However, the crude product shows in its FAB-mass spectrum an intense peak (at 60% relative intensity), attributable to the molecular ion of an oxidized product, [Mo3ReS4(O)2(dtp)5]+ (2+), together with the expected peaks from [Mo3ReS4(dtp)6]+ (m/e 1714, 65%), [Mo3ReS4(dtp)5]+ (m/e 1528, 100%) and [Mo3ReS4(dtp)4]+ (m/e 1357, 45%). Attempts to recrystallize the crude product from an acetone-methanol mixture in air lead to complete oxidation of 1 into 2, which separates as blue single crystals of X-ray quality.
The change in reactivity towards oxidation on going from oxidatively very stable Group 6 metal-only dithiophosphates, [M4Q4(dtp)6] (M = Mo, W; Q = S, Se), to Re-containing cluster cores is striking [21]. Introduction of rhenium atom may perturb electron count and electron density distribution, but it is the molybdenum which is really oxidized, as was revealed by X-ray structural analysis.
Fig. 1 shows the ORTEP representation of the neutral complex 2 with the atom numbering scheme. Cluster 2 crystallizes in the C2/c space group and has a crystallographically imposed C2 symmetry where the half of each molecule is related by the transformation –x + 1, y, –z + 3/2 as shown in Fig. 1. Table 1 shows the most relevant bond distances and angles for compound 2.
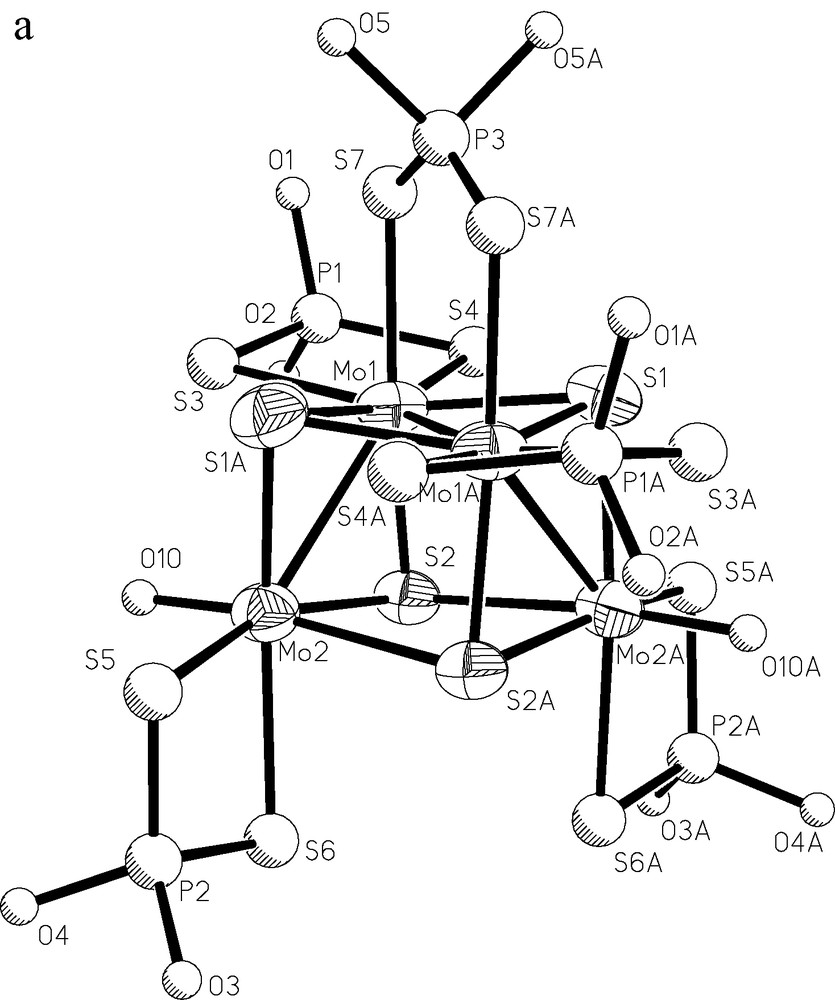
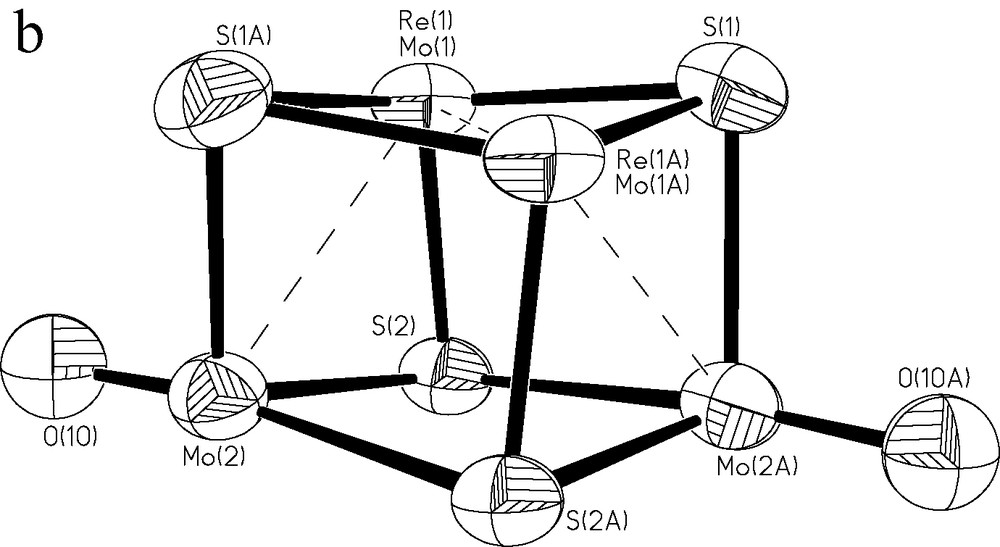
(a) Partial ORTEP representation of compound 2 with the atom-numbering scheme. All atoms except those belonging to the cluster core have been drawn as spheres for clarity. (b) Cuboidal arrangement of the central cluster core in 2, where dashed lines emphasize the zig-zag disposition of the metals.
Selected bond distances (Å) and angles (°) in cluster 2
| Mo(1)/Re(1)–Mo(1A)/Re(1A) | 2.6649(16) | Mo(2)–O(10) | 1.696(8) |
| Mo(1)–Mo(2) | 2.8073(13) | Mo(2)–S(2A) | 2.703(3) |
| Mo(1)–S(1A) | 2.298(3) | Mo(2)–S(2) | 2.340(3) |
| Mo(1)–S(1) | 2.414(4) | Mo(2)–S(1A) | 2.369(3) |
| Mo(1)–S(2) | 2.338(3) | ||
| S(1A)–Mo(1)–S(2) | 106.93(11) | S(2)–Mo(2)–S(2A) | 82.26(10) |
| S(1A)–Mo(1)–S(1) | 109.72(11) | S(1A)–Mo(2)–S(2A) | 86.45(10) |
| S(2)–Mo(1)–S(1) | 94.23(11) | O(10)–Mo(2)–Mo(1) | 100.9(3) |
| S(1A)–Mo(1)–Mo(1A) | 57.64(9) | S(2)–Mo(2)–Mo(1) | 53.08(7) |
| S(2)–Mo(1)–Mo(1A) | 97.42(7) | S(1A)–Mo(2)–Mo(1) | 51.87(9) |
| S(1)–Mo(1)–Mo(1A) | 53.53(9) | S(2A)–Mo(2)–Mo(1) | 86.18(7) |
| S(1A)–Mo(1)–Mo(2) | 54.19(8) | Mo(1A)–S(1)–Mo(2A) | 73.94(10) |
| S(2)–Mo(1)–Mo(2) | 53.16(7) | Mo(1A)–S(1)–Mo(1) | 68.83(10) |
| Mo(1A)–Mo(1)–Mo(2) | 75.35(3) | Mo(2A)–S(1)–Mo(1) | 88.80(10) |
| O(10)–Mo(2)–S(2) | 100.1(3) | Mo(1)–S(2)–Mo(2) | 73.76(8) |
| O(10)–Mo(2)–S(1A) | 99.7(3) | Mo(1)–S(2)–Mo(2A) | 82.86(9) |
| S(2)–Mo(2)–S(1A) | 104.56(11) | Mo(2)–S(2)–Mo(2A) | 96.63(10) |
| O(10)–Mo(2)–S(2A) | 172.6(3) |
The cluster core in 2 can be described as belonging to the well-known cuboidal type, in our case represented by new Mo3ReS49+ core. Both Mo(1)/Re(1) and Mo(2) metallic positions have a pseudo-octahedral environment defined by three bridging sulfur atoms of the cluster core, one chelating dtp ligand and one terminal oxygen atom O(10) on the Mo(2) site, or two sulfur atoms of the bridged dtp ligand on the Mo(1) site. This dtp ligand connects crystallographically equivalent Mo(1)/Re(1) atoms. The Mo(2)–O(10) bond distance of 1.679(9) Å is consistent with the presence of a Mo=O double bond. This value is very close to that found in a related cuboidal cluster which also contains a double Mo=O bond, namely [Mo3(CuI)S4(O)2(C4H8NCS2)5] [17].
Due to the electron deficiency, the structure of 2 results in a distorted zig-zag arrangement of metal atoms rather than the most common tetrahedral connectivity observed in the vast majority of clusters with Mo3M′S4 cores [21]. The latter require 12 metal electrons to form six 2c–2e– metal–metal bonds, while in 2 only eight electrons are available. Fig. 1b) emphasizes the zig-zag disposition of the metallic core. This arrangement has three metal–metal bonds where the Mo(2) atoms occupy the peripheric positions and the Mo(1)/Re(1) atoms occupy the hinge positions. All four sulfur atoms in the cluster core are triply bridging but two out of the twelve Mo(Re)-μ3-S bonds are rather weak (2.701(3) Å). The Mo(1)/Re(1)-Mo(1A)/Re(1A) bond distance of 2.665(2) Å is short for a single bond [21] while the two Mo(1)/Re(1)–Mo(2) bond lengths of 2.8074(14) Å fall within the single bond range. The remaining molybdenum–molybdenum bond distances, namely Mo(1)–Mo(2A), Mo(2)–Mo(1A) and Mo(2)–Mo(2A), (3.346(2)–3.774(3) Å) are clearly beyond any significant metal–metal bonding.
Taking into account the observed M–M and M–S distances, the structure can be rationalized in the following way. The Mo(2) and Mo(2A) atoms have all the structural features of MoO3+ complexes, including weak bonding trans to the Mo=O group (well-known trans-effect of multiple bonds). This would give us two identical {Mo(O)(S)(dtp)} building blocks, which ‘condense’ with the central unit {ReMo(S)2(dtc)2}(reversible dissociation of other highly oxidized cuboidal clusters was indeed observed) [21]. This central unit is in fact a d3–d3 edge-sharing bioctahedral complex, with the most probable electronic configuration σ2π2δ*2 [18,19]. Upon cluster formation the electrons from the δ* orbital form two single M–M bonds leaving the central Mo = Re bond essentially double, in a good agreement with the observed short bond distance. The reason why other clusters with only eight metal electrons for M–M bonding (such as Cp4V4S4, Nb4Se4I4) do not show this kind of disruption of the metal tetrahedron, preferring instead electron delocalization with overall long but almost equal M–M distances, is the high symmetry of their external ligand environment [20]. The bonding situation in the metal skeleton of [Mo3(CuI)S4(O)2(C4H8NCS2)5] is different, leading to an ‘umbrella’-type arrangement with three terminal M–M bonds (two Mo–Mo and one Mo–Cu) issuing from one central Mo atom [17]. However, the uncertainty about the extent of real Cu participation in the M–M bonding does not allow meaningful comparison of this compound with 2 in the absence of theoretical calculations.
Acknowledgements
Financial support from Ministerio de Ciencia y Tecnología (research project BQU2002-00313) is gratefully acknowledged. M.S. is grateful to the Russian Science Support Foundation for a grant.


