1 Introduction
Recently the dye-sensitized solar cell (DSSC) [1] has attracted much attention around the world due to its high light-to-electricity conversion efficiency (10.4%) and easy fabrication [2]. More importantly, the low cost of DSSC makes it attractive for commercialization. However, the liquid electrolyte is still a “threat” for DSSCs’ practical application because many problems regarding liquid electrolyte remain unresolved, such as solvent evaporation, leakage and deterioration, which causes difficulties in sealing and performance degradation of DSSCs.
Solid-state electrolytes or quasi-solid-state electrolyte such as crystalline p-type semiconductors [3–7], hole-conducting molecular solids and polymers [8–15] and molten salts or ionic liquids [16–21] have been investigated and employed to fabricate DSSCs to substitute the conventional volatile organic solvent-based electrolyte. But most of results turn out to be unsatisfactory because of poor performance of DSSC in practical use.
Some addition compounds formed by LiI and organics have been reported as electrolytes [22–26]. The preparation of LiI(CH3OH)4, a solid-state electrolyte, is very simple and its ionic conductivity is relatively high [22], which indicates its potential use in DSSCs. In our experiments, we use this simple solid-state electrolyte and iodine to form the redox couple in DSSCs. THT is added as cooperative electrolyte to facilitate the filling of LiI(CH3OH)4–I2 into the pores of TiO2 films and SiO2 particles are included to inhibit the crystal growth of LiI(CH3OH)4. Meanwhile, SiO2 particles can form an insulating layer between the TiO2 film and the counter electrode. A high energy conversion efficiency is achieved by using this composite electrolyte in DSSCs.
2 Experimental section
LiI was purchased from Acros and used without further treatment. CH3OH was HPLC grade (Concord Tech, Tianjin, China) and used as received. LiI(CH3OH)4 solid-state electrolyte was prepare by adding CH3OH into LiI powder (LiI/CH3OH = 1:4, molar) in an argon-filled glove box (M. Braun Company). The water content is less than 0.1 ppm. A great amount of heat was released during the reaction of LiI and CH3OH while vigorously stirring and the LiI(CH3OH)4 compound was formed.
The preparation of triethylamine hydrothiocyanate (THT) was described in literature [27] and was added in the electrolyte in the argon-filled glove box.
Three kinds of electrolyte solutions were prepared: (a) 0.6 g LiI(CH3OH)4 and 0.009 g I2 (YiLi Fine Chemicals, Beijing, China, LiI(CH3OH)4/I2 = 100:1, molar) were dissolved in 7.2 ml dimethyl carbonate(DMC, battery grade, PhyLion Co. Ltd. Beijing, China); (b) 0.6 g LiI(CH3OH)4, 0.009 g I2, 0.06 g SiO2 nano-particles (Degussa, particle diameter: 14 nm) were dissolved in 7.2 ml of DMC and then sonicated for 1 h and stirred overnight; (c) 0.6 g LiI(CH3OH)4, 0.009 g I2, 0.06 g SiO2 nano-particles and 0.06 g THT were dissolved in 7.2 ml of DMC and sonicated for 1 h; then stirred overnight. About 0.45 ml of 4-tert-butyl-pyridine (Aldrich) [2,14] was also added into each solution.
The TiO2 porous film deposition on F-doped tin oxide(FTO) conducting glass (12 Ω □–1) and the dye (RuL2(NCS)2·2H2O, L = 2,2′-bipyridyl-4,4′-dicarboxylic acid, Solaronix) adsorption were carried out according to Ref. [2]. The thickness of the TiO2 film we prepared was about 10 μm. The dye-anchored TiO2 film was put on a hot plate at 40 °C. The electrolyte solution was dropped onto the film by a pipet. Then the film was put on the hot plate for 5 min. The solvent (DMC) evaporated quickly and, in order to eliminate the solvent residue, the film was placed in a vacuum chamber for 3 min. The same procedure was repeated several times until an even film of electrolyte was formed on the TiO2 film. Averagely, about 4 mg cm–2 of electrolyte was deposited on the TiO2 film. A platinum-sputtered conducting glass plate was clipped firmly with the TiO2/dye/electrolyte glass plate. A window of 0.18 cm2 was also clipped on the TiO2 side to define the active area of the cell.
The cells were illuminated by an Oriel solar simulator (91192) under AM 1.5 (92 mW cm–2) irradiation. The incident light intensity was measured by a radiant power/energy meter (Oriel 70260). The I–V characteristics of the cells were recorded by a potentiostate/galvanostate (Princeton Applied Research, Model 263A).
3 Results and discussion
Fig. 1a shows the I–V curve of the DSSC assembled using only LiI(CH3OH)4–I2. The open-circuit voltage, short-circuit photocurrent density, fill factor and overall efficiency are 0.62 V, 2.8 mA cm–2, 0.58% and 1.1%, respectively. The poor performance of the cell can be ascribed to the large agglomerations of LiI(CH3OH)4 that cannot completely fill into the porous TiO2 films.
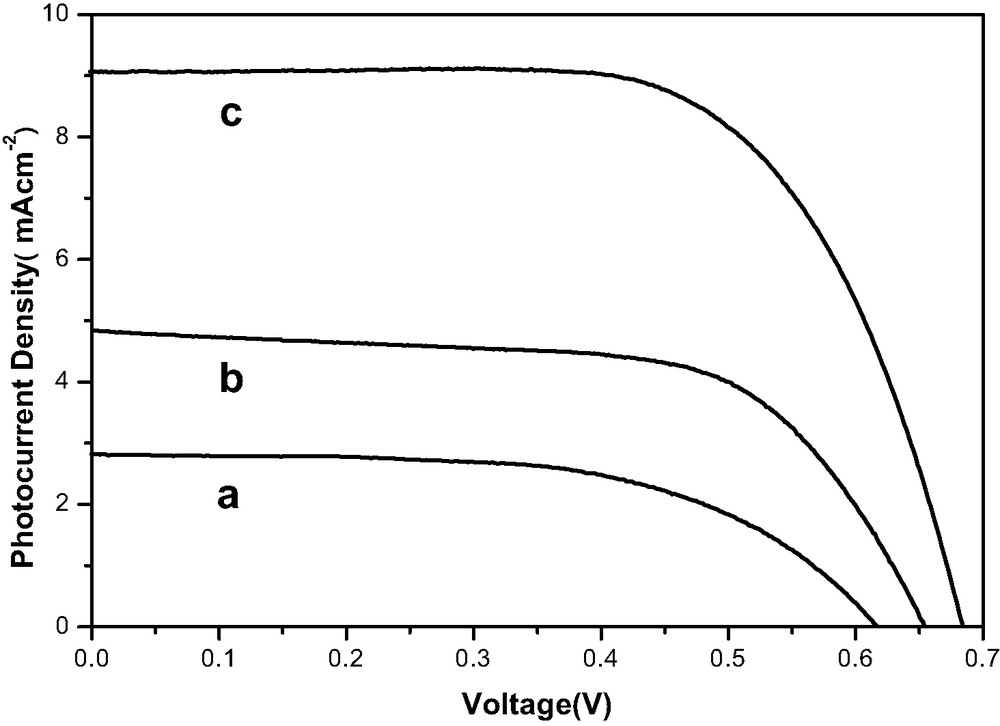
The I–V characteristics of dye-sensitized solar cells with electrolytes.
(a) LiI(CH3OH)4–I2.
(b) LiI(CH3OH)4–I2 and SiO2 nano-particles.
(c) LiI(CH3OH)4–I2, THT and SiO2 nano-particles.
Fig. 1b shows the I–V curve of the DSSC assembled using LiI(CH3OH)4–I2 and SiO2 particles. The open-circuit voltage, short-circuit photocurrent density, fill factor and overall efficiency are 0.66 V, 4.8 mA cm–2, 0.63% and 2.17%, respectively. As can be seen from the I–V curve, the performance of DSSCs is increased remarkably by adding SiO2 particles into the electrolyte. We attribute the great performance improvement to two reasons: one is that the small electrolyte crystals can readily fill the voids of the TiO2 films and have better contact with the dye molecules; Another reason is the thin insulating layer formed by the SiO2 particles, as we observed in the experiments after the TiO2 film was treated in the vacuum chamber, between the two electrodes of the cell, which reduces the possibility of short-circuiting of the cell.
Fig. 1c shows the I–V curve of the DSSC assembled using LiI(CH3OH)4–I2, SiO2 particles and THT. The open-circuit voltage, short-circuit photocurrent density, fill factor and overall efficiency are 0.68 V, 9.1 mA cm–2, 0.66% and 4.43%, respectively. By adding SiO2 and THT simultaneously, the energy conversion efficiency is increased by more than 100% compared to those cells using LiI(CH3OH)4–I2–SiO2. We explain this great improvement by the cooperative effect of LiI(CH3OH)4–I2 and THT: when THT is added into the LiI(CH3OH)4–I2–SiO2 system, THT can penetrate into the void left by the LiI(CH3OH)4 crystallites, so the two electrolytes can form continuous phase over the TiO2 films. Thus, the contacts between the electrolyte layer, the dye-anchored TiO2 film and the counter electrode have been improved and the energy conversion efficiency increases accordingly. In our experiments, we find that other ionic liquids have the same effect as THT [26].
4 Conclusions
A DSSC with high-energy conversion efficiency of 4.43% is successfully assembled using a simple solid-state electrolyte, LiI(CH3OH)4, with adding SiO2 and THT at the same time. The two additives improve the interface contacts in the solar cell, which is of vital importance for current collection in DSSCs. The thin insulating layer formed by SiO2 particles also plays an important role in reducing the possibility of short-circuiting of DSSC.
Acknowledgements
We acknowledge the support of the National 863 program of China (Contact No. 2002AA302403), the ‘100-talent’ project of Chinese Academy of Sciences and the New Energy and Industrial Technology Development Organization (NEDO) of Japan.


