1 Introduction
In recent years, much attention has been focused on solar photovoltaic generation as a renewable energy source. Dye-sensitized solar cells (DSCs) [1] are expected to be an alternative candidate for conventional silicon solar cells because of their simple fabrication process and low cost of raw materials.
Improvement in conversion efficiency and long-term stability of DSC is a minimum requirement for practical application of DSCs. Instability of DSC is attributed to evaporation and leakage of liquid electrolyte. In order to solve these problems ionic liquid electrolytes prepared from ionic liquid (room temperature molten salt), nonvolatile solvent and ionic gel electrolytes obtained by gelation of ionic liquid electrolyte have been developed. Many kinds of materials, organic polymer [2–4], hydrogen bond type organic molecule [5] and inorganic nano particles [6–8], have been investigated as gelators for ionic gel electrolytes of DSC.
Agarose, a polysaccharide, is frequently used as a gelator of aqueous electrolytes. A remarkable feature of agarose is its ability to form a mechanically strong gel with a small amount of agarose while keeping the ionic conductivity of the liquid electrolyte almost unaffected [9,10]. However, but few attempts have been made to investigate gel electrolytes prepared from ionic liquid and agarose. This is because solubility of polysaccharides in an ionic liquid is generally low and gel formation is difficult. Until now only a few studies have reported research on ionic liquids that show solubility of polysaccharides. One example is imidazolium bromide containing an ether substitute [11] and another is molten salt of imidazolium chlorides at high temperatures [12].
We have found that a novel ionic gel can be prepared from agarose and 1-alkyl-3-methylimidazole iodides (Scheme 1). Here we report the properties of this ionic gel and cell performance characteristics of DSC containing ionic gel electrolytes.
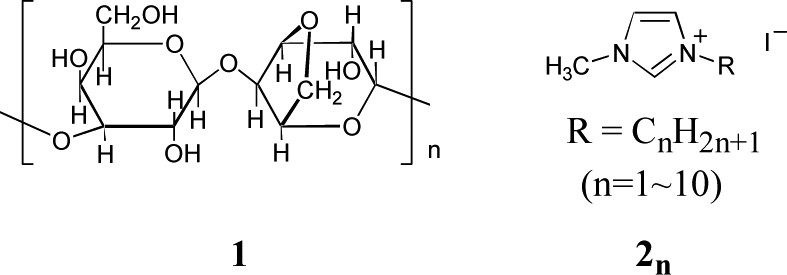
Chemical structure of agarose (1) and 1-alkyl-3-methylimidazolium iodide (2n).
2 Experimental section
Ionic liquids, 1-alkyl-3-methyl-imidazolium iodides (2n, n = 1–10) [13], 1-methyl-3-propylimidazolium hexafluorophosphate (MPIm-PF6) [13], 1-ethyl-3-methylimidazolium dicyanamide (EMIm-DCA) [14], 1-ethyl-3-methylimidazolium tetrafluoroborate (EMIm-BF4) [13] and 1-ethyl-3-methylimidazolium bis(trifluoromethanesulfon)imide (EMIm-TFSI) [13], were prepared by procedures described previously.
Gel formation ability was examined by the following method, 1.5 wt% of agarose (LO3, Takara Bio) was poured into ionic liquids and the mixtures were stirred at 150 °C for 10 min. After the solutions were cooled to room temperature, appearance of the solution was observed.
Compositions of electrolytes were as follows (Table 1). Liquid electrolyte: 3-methoxypropionitrile (MPN) solution of iodine (I2, 0.05 M, M = mol dm−3), lithium iodide (LiI, 0.10 M), 4-tert-butylpyridine (TBP, 0.50 M) and 1,2-dimethyl-3-propylimidazolium iodide (DMPIm-I, 0.30 M, Shikoku Corp.). Ionic liquid 1 (IL1) electrolyte: 1-methyl-3-propylimidazolium iodide (MPIm-I, 23 Scheme1) solution of I2 (0.35 M), LiI (0.10 M) and TBP (0.50 M). Ionic liquid 2 (IL2) electrolyte: MPIm-I solution of I2, (0.35 M), LiI (0.02 M) and TBP (0.50 M). Ionic gel electrolyte: MPIm-I solution of I2, (0.35 M), LiI (0.02 M), TBP (0.50 M) and agarose (1.0 wt%).
Composition of liquid, ionic liquid and ion gel electrolyte
| Electrolytea | Solventb | I2 | LiI | TBPc | DMPIm-Id | Agarose |
| Liquid | MPN | 0.05 | 0.10 | 0.50 | 0.30 | — |
| IL1 | MPIm-I | 0.35 | 0.10 | 0.50 | — | — |
| IL2 | MPIm-I | 0.35 | 0.02 | 0.50 | — | — |
| Ionic gel | MPIm-I | 0.35 | 0.02 | 0.50 | — | 1wt% |
a IL1: ionic liquid 1, IL2: ionic liquid 2.
b MPN: 3-methoxypropionitrile. MPIm-I: 1-methyl-3-propylimidazolium iodide.
c TBP: 4-tert-butylpyridine.
d DMPIm-I: 1,2-dimethyl- 3- propylimidazolium iodide. Unit of the concentration of I2, LiI, TBP and DMPIm-I is mol dm−3.
Method for fabrication of the TiO2 photo-electrodes is outlined below. TiO2 colloidal paste (Ti-Nanoxide T, particle size: 13 nm, Solaronix) was spread on a fluorine-doped tin oxide conducting glass electrode (FTO, U-film, Asahi Glass). A mixed suspension of ZrO2 (0.050 g, JRC-ZEO-1: particle size 36 nm), TiO2 (0.45 g, JRC-TIO-2: particle size 400 nm, JRC-ZEO-1 and JRC-TIO-2 were reference catalysts obtained from the Catalysis Society of Japan), cellulose polymer (0.10 g, Marpolose 60MP-50, Matsumoto Yushi Seiyaku), polyethylene glycol (0.30 g, Mw 20000, Wako) and water (5 ml) was applied to the TiO2 layer as a light reflection layer. After sintering at 450 °C for 30 min, the TiO2 electrodes were colored with ruthenium dye [15] (N719, Solaronix). Thickness of the TiO2 layer was 5 μm and that of the light reflection layer was 2 μm.
Sealed and unsealed solar cells were assembled by the following way. Unsealed cell: electrolyte was spread on the TiO2 electrode. The TiO2 electrode was placed on a counter electrode (Pt sputtered FTO) and fixed with a holder. Sealed type cell: TiO2 electrode was affixed to a counter electrode with a thermal adhesive film (Himilan, Du Pont-Mitsui polychemicals) of 30-μm thickness. Electrolyte was poured into the cell through a small hole in the electrode and the hole was sealed with the same thermal adhesive film.
Photocurrent-voltage curves were measured at room temperature (20–25 °C) under simulated sunlight (AM 1.5, 100 mW cm−2). The active area of the TiO2 photo-electrode was 0.25 cm2.
3 Results and discussion
3.1 Preparation and properties of the ionic gel
The ability of agarose to form a gel in ionic liquid was examined. Appearance of a mixture of ionic liquid and agarose was observed after stirring at 150 °C for 10 min. Five kinds of 1-alkyl-3-methylimidazolium salts, MPIm-I (23; Scheme 1), MPIm-PF6, EMIm-DCA, EMIm-BF4 or EMIm-TFSI were used as ionic liquids. Agarose formed a gel with MPIm-I, MPIm-PF6 and EMIm-CDA. Especially, a strong gel was obtained from agarose and MPIm-I.
Next, gel formation ability was also examined with other alkylimidazolium iodides, 2n (n = 1–10), which contained a series of alkyl groups (R = CnH2n+1, n = 1–10). Ionic gels were obtained from six different imidazolium iodides (23, 24, 25, 26, 27, 28) containing alkyl groups of 3 to 8 carbons. In the imidazolium iodide with 1 or 2 carbons (21, 22), agarose dissolved at 150 °C. However, the solutions crystallized at room temperature because the melting points of these salts were above room temperature. Agarose did not completely dissolve in the 29 and 210 imidazolium iodides because the hydrophilicity of 29 and 210 become lower with an increase in the alkyl chain length.
Fig. 1 shows the appearance of ionic gel prepared from 1-methyl-3-propylimidazolium iodides (23) and agarose. The gel was a transparent elastic material similar to rubber. It could be picked up with tweezers and the shape changed with external force. The gel did not flow when placed on an inclined surface for a long time (at least one year) but ionic liquid gradually leaked out from the gel when placed on a fibrous or porous material like paper. These properties show immobility of the ionic gel and the possibility of migration of solution inside the ionic gel. The gel becomes softer at high temperatures. The gel of 23 with 1.5 wt% agarose melted at approximately 120 °C.

Appearance of ionic gel prepared from 1-propyl-3-methylimidazoloum iodide (23) and agarose (1.5 wt %).
3.2 Preparation of ionic gel electrolyte
Ionic liquid electrolytes and ionic gel electrolytes were prepared from MPIm-I (23) and agarose. A liquid electrolyte using organic solvent (3-methoxypropionitrile: MPN) was also prepared as a standard electrolyte. Compositions of the electrolytes are shown in Table 1. Lithium iodide (LiI) was added to the ionic liquid 1 (IL1) electrolyte (Table 1) at a concentration of 0.10 M (M = mol dm−3) in order to increase photocurrent of the solar cell. Agarose did not dissolve in IL1 electrolyte because LiI reduced the solubility of agarose. Ionic gel electrolyte was prepared by addition of agarose to IL2 electrolyte in which the concentration of LiI was decreased from 0.10 M to 0.02 M.
3.3 Properties of DSC with ionic gel electrolyte
Conversion efficiency (η) was measured at room temperature (20-25 °C) under standard conditions (AM 1.5, 100 mW cm−2) using an unsealed solar cell (open cell). Efficiency of IL1, IL2 and ionic gel electrolyte were 3.01, 2.77 and 2.41%, respectively (Table 2). IL2 electrolyte showed a slightly lower efficiency as compared to IL1 electrolyte. This is due to a lower concentration of LiI (0.02 M) in IL2 electrolyte. Ionic gel electrolyte further showed low efficiency when compared to IL2 electrolyte, but the difference was small and 87% of the efficiency of IL2 electrolyte was maintained.
Comparison of conversion efficiency and electrode distance in unsealed and sealed solar cells
| Unsealed cell | Sealed cell | |||
| Electrolyte | η / % | d / μm | η / % | d / μm |
| IL1 | 3.01 | 7 | 2.90 | 30 |
| IL2 | 2.77 | 7 | 2.51 | 30 |
| Ionic gel | 2.41 | 35 | 2.46 | 30 |
Distance between the TiO2 electrode and counter electrode of the cell with IL1 and IL2 electrolyte was about 7 μm (sum of TiO2 layer and light reflection layer) but the distance of the cell with ionic gel electrolyte was much larger (35 μm, Table 2). This is attributed to the fact that the ionic gel electrolyte was difficult to apply on the TiO2 electrode as a thin film because the gel was a semi-solid. Distance between electrodes should be kept at uniform conditions because thickness of the electrolyte affects performance of the cell [16]. Next measurement was carried out using a sealed solar cell (closed cell) in which the electrode was fixed with adhesive film at 30-μm thickness. Under this condition, efficiency of the cell with of IL1, IL2 and ionic gel electrolyte were 2.90, 2.51 and 2.46%, respectively (Table 2). Efficiency of IL1 and IL2 decreased when compared to the unsealed cell owing to an increase in electrolyte thickness. However, the ionic gel electrolyte maintained almost the same efficiency even in the sealed type cell. Efficiency of the ionic gel electrolyte was 98% that of IL2 electrolyte. From the above results, it is apparent that the ionic gel electrolyte maintains the same ability as IL2 electrolyte.
Time dependence of conversion efficiencies in sealed type solar cells are shown in Fig. 2. Changes in the curvature of the lines were almost the same in all electrolytes. Efficiencies increased about 10-15% of the initial efficiency in the first 20 h and a small increase was also observed during the next 300 h. This was attributed to slow penetration of ionic liquid electrolytes into the nano-meter sized porous TiO2 electrode because viscosity of MPIm-I (865 mPa s at 25 °C [5]) was much larger than that of organic solvents (MPN: 1.1 mPa s at 25 °C [17]).

Time dependence of conversion efficiency of sealed solar cell with ionic liquid and ionic gel electrolyte. IL1: ionic liquid 1, IL2: ionic liquid 2.
Cell performance characteristics at 310 hours are summarized in Table 3. Efficiency of the cell with IL1, IL2 and ionic gel electrolyte were 3.23, 2.95 and 2.93%, respectively. Under the same electrolyte conditions, these values were larger than the efficiency of the unsealed solar cell. This result clearly shows the importance of electrolyte penetration into the TiO2 electrode in order to obtain a highly efficient solar cell.
Cell performance characteristics of sealed solar cells with liquid, ionic liquid and ionic gel electrolytes at 310 hours
| Electrolyte | Voc / V | Jsc / mA cm−2 | FF | η / % |
| Liquid | 0.800 | 10.28 | 0.677 | 5.57 |
| IL1 | 0.670 | 7.79 | 0.619 | 3.23 |
| IL2 | 0.675 | 6.55 | 0.668 | 2.95 |
| Ionic gel | 0.650 | 6.77 | 0.666 | 2.93 |
Voltage (V) dependence of current density (J) of cells with IL2 and ionic gel electrolyte at 310 hours are shown in Fig. 3. Though small differences were observed, almost the same J–V curves were obtained.

Photocurrent density–voltage curves of sealed type solar cells with ionic liquid 2 (IL2) and ionic gel electrolytes at 310 h.
In this study, thickness of the electrolyte layer of a sealed solar cell was kept at 30 μm. Conversion efficiency of the cell can be improved by making the electrode layer thinner. Improvements in the manufacture methods to assemble thin electrolyte solar cells are currently in progress.
3.4 Ionic-gel electrolyte with EMIm-DCA and agarose
EMIm-DCA and MPIm-PF6 solutions of agarose also form ionic gels. EMIm-DCA-based ionic-gel electrolyte was prepared from an EMIm-DCA solution of 0.35 M I2, 0.02 M LiI, 0.50 M TBP, 1.05 M DMPIm-I and 3.0 wt% agarose. This electrolyte showed a conversion efficiency (η) of 3.89%, short circuit photocurrent density (Jsc) of 7.93 mA cm−2, open-circuit voltage (Voc) of 0.830 V and fill factor (FF) of 0.591. MPIm-PF6 based ionic gel electrolyte was also prepared but 1.0 wt% of agarose did not completely dissolve in the MPIm-PF6 solution of 0.35 M I2, 0.02 M LiI, 0.50 M TBP, 1.05 M DMPIm-I and a gel electrolyte was not obtained. These are preliminary results without optimization. Further studies using a binary gel electrolyte with MPIm-I and EMIm-DCA is currently under way.
4 Conclusion
Agarose can be dissolved in 1-alkyl-3-methylimidazolium iodides (23, 24, 25, 26, 27, 28), EMIm-DCA and MPIm-PF6 at a high temperature (150 °C). These agarose solutions form ionic gels at room temperature. Lithium iodide reduces the solubility of agarose in ionic liquids but ionic gel electrolytes can be prepared by reduction of LiI concentration in the ionic liquid electrolyte. According to this, semi-solid ionic gel electrolytes which maintain the same ability as ionic liquid electrolytes can be prepared from MPIm-I (23) and agarose. Conversion efficiency of the solar cell with ionic liquid and ionic gel electrolyte are largely affected by cell distance and penetration of electrolytes into the TiO2 electrode.
Acknowledgments
This work was supported by the New Energy and Industrial Technology Development Organization (NEDO) under the Ministry of Economy Trade and Industry (METI). We acknowledge Shikoku Corporation, Matsumoto Yushi Kasei and Du Pont-Mitsui polychemicals for respectively supplying 1,2-dimethyl-3-propylimidazolium iodide, cellulose polymer and thermal adhesive film.


