1 Introduction
Dye-sensitized solar cells (DSCs) continue to attract much attention as viable systems for conversion of solar energy [1–4]. The highest efficiency achieved is of the order of 10% for a TiO2 based cell sensitized with RuN3 dye [1]. An increase of the open-circuit voltage by suppression of recombination could increase the efficiency by an additional few percent, however, in order to increase the efficiency of DSCs to a level comparable to that of the silicon solar cell, broadening of the spectral response become an essential requirement. DSCs have narrower spectral response corresponding to the absorption spectrum of the dye. Synthesis of dyes with broader spectral response has been attempted as a possible strategy. The other method is to use more than one pigment. Unfortunately, the straightforward way of doing this by using mixtures of dyes is unsuccessful owing to the quenching and insulating effect of thick dye layers composed of many components [5]. Direct application of dye mixtures, in almost all cases result lowering of both energy and quantum conversion efficiencies compared to at least that of the cell based on the best single dye. If several dyes are homogeneously dispersed over the nanocrystalline surface at sub-monolayer coverage of each dye, the problem of quenching and insulation may be avoided. This leads to another constraint involving material properties of the semiconductor film. In order to achieve full light absorption at the peak wavelength of each dye, the film thickness T needs to be increased, the limit for which is the diffusion length L = (D τ)1/2, where D = diffusion coefficient and τ = recombination time. Suppose we use two dyes of same ‘molecular area’, in order to satisfy the constraint T ≤ L, a fourfold increase in the diffusion coefficient is necessary (as the roughness factor is proportional to film thickness). This is hard to achieve, because the diffusion coefficient depends on the film material and film morphology and attempts to adjust film morphology would also change the roughness factor.
We have found some ways of circumventing the above difficulties and construct model dye-sensitized solid-state solar cells [6,7] (DSSSCs) with more than one pigment, showing energy and quantum conversion efficiencies above that of the cells sensitized with the individual dyes. One method we adopted was to separate the two dye layers by a barrier of a high band-gap semiconductor or an insulator [5]. Here a monolayer of dye D1 coated on TiO2 is followed by an ultra-thin barrier of p-CuSCN (denoted by p-CuSCN) and the outer surface of p-CuSCN is coated with the second dye D2 and followed by a thick layer of p-CuSCN to form the heterojunction n-TiO2/D1/p-CuSCN/D2/p-CuSCN. Choosing D1 = Fast Green and D2 = Acridine Yellow, we could show that the energy and quantum conversion efficiency of double dye system is higher than that of the cells n-TiO2/D1/p-CuSCN or n-TiO2/D2/p-CuSCN. The difficulty of this technique is making the barrier sufficiently thin and at the same time preventing formation of pinholes. The other method, we attempted was to bond anionic (cationic) dye D1 covalently to TiO2 by suitable ligands and electrostatically couple a cationic dye (anionic dye) D2 to D1 to form the heterostructure n-TiO2/D1–D2/p-CuSCN. Here again, with suitable choices for D1 and D2 (i.e. D1 = mercurochrome, D2 = methyl violet) we succeeded in demonstrating that the double dye system has higher energy and quantum conversion efficiencies compared to cells based on individual dyes [8]. In this paper, we discuss the possibilities of constructing efficient dye-sensitized solid-state solar cells by coupling two dyes to each other and to n and p-type semiconductors (N and P) to form the heterojunction N/D1–D2/P.
2 Experimental
Nanocrystalline films of TiO2 of the type suitable for constructing DSSSCs were deposited on conducting fluorine doped tin oxide glass plates (0.5 × 1.5 cm2, active area = 0.25 cm2) by the method described previously [9] Briefly, the procedure involves spreading of a colloidal solution of titanium dioxide (prepared by hydrolysis of titanium isopropoxide in the presence of acetic acid) on CTO glass plates heated to 150 °C and sintering at 450 °C in air for 30 min. After cooling, the loose crust on the surface is removed and the process is repeated until the film thickness reaches ~ 10 μm. Films prepared by the above method are largely free of interconnected pores leading to the back contact and have roughness factors in the order 200–300. Films are washed with propan-2-ol, dried and exposed to UV light for 20 min to burn out any organic contamination on the surface. Dyes used in this investigation are bromopyrogallol red (BR), mercurochrome (MC), methyl violet (MV), and IR786 purchased from Aldrich .The anionic dyes BR and MC that get readily adsorbed on the TiO2 surface were coated on the nanocrystalline film by soaking it in an alcoholic solution (~ 10−3 M) of the dye for about one hour. The cationic dyes (MV, BR or IR786) were deposited on anionic-dye-coated surface by exposing this film to a solution of the cationic dye in 75% alcohol. The amounts dyes adsorbed on the film was estimated by extraction of the dyes into the alkaline alcoholic solution and spectrophotometric estimation after adjustment of pH (sample plates of identical batch were used). The heterojunctions n–TiO/D1–D2/p-CuSCN, where D1 = anionic dye BR or MC and D2 = cationic dye MV, or IR 786 were formed by depositing p-CuSCN from a solution of propyl sulfide [10] over the structure n-TiO2/D1–D2. Graphite was painted on the outer surface of CuSCN, and a gold plated CTO glass plate pressed onto to graphite served as the back contact. A schematic diagram showing the construction of the cell is presented in Fig. 1.

Schematic diagram showing the construction of the cell n-TiO2/D1–D2/p-CuSCN.
I-V characteristics of the cells were recorded with a Keithley 2420 Source Meter and a xenon lamp at intensity 1000 Wm−2 as the light source. Photocurrent action spectra were recorded using a Nikon (G 250) monochromator and light intensities were measured with an Eko Pyranometer.
3 Results and discussion
Different possible configurations for double-dye DSSSCs are presented in Fig. 2. In the configuration shown in Fig. 2a, the dye layer is homogeneously mixed and thick Here it is unlikely that excitation of every D1 or D2 molecule will lead to efficient vectorial injection of electrons to N region and holes to the P region and the quenching processes, i.e:
| (1) |
| (2) |
| (3) |

Schematic diagrams illustrating possible configurations of double-dye solid-state solar cells, (a) homogenously mixed thick-layer two-dyes cell, (b) a monolayer consisting of two non-interacting dye molecules coupled to n and p-type semiconductors, (c) dye layer consisting of two electronically coupled dye molecules bonded on opposites sides to n and p-type semiconductors (circles indicate two types of dye molecules).
In the situation depicted in Fig. 2.(b), dye molecules do not interact with each other but each molecule is anchored to both N and P- type semiconductors. As the dye molecules are non-interacting, the quenching processes will be absent. However, the coverage of each dye will be at sub-monolayer level and the absorption cross-section of light at two peak wavelengths will be below the optimum. In the configuration shown in Fig. 2c, the two dye molecules D1 and D2 are coupled to each other and also anchored to N and P regions respectively. If the location of the ground (So) and excited (S*) levels of the dye relative to the two semiconductors are as in Fig. 3, excitation of D1 could result electron injection to N region and hole injection P region. The latter process involves hole conduction through the molecule D2. Similarly, excitation of D2 could result hole injection to P region and electron injection to N region via conduction through the dye molecule D1, i.e:
| (4) |
| (5) |
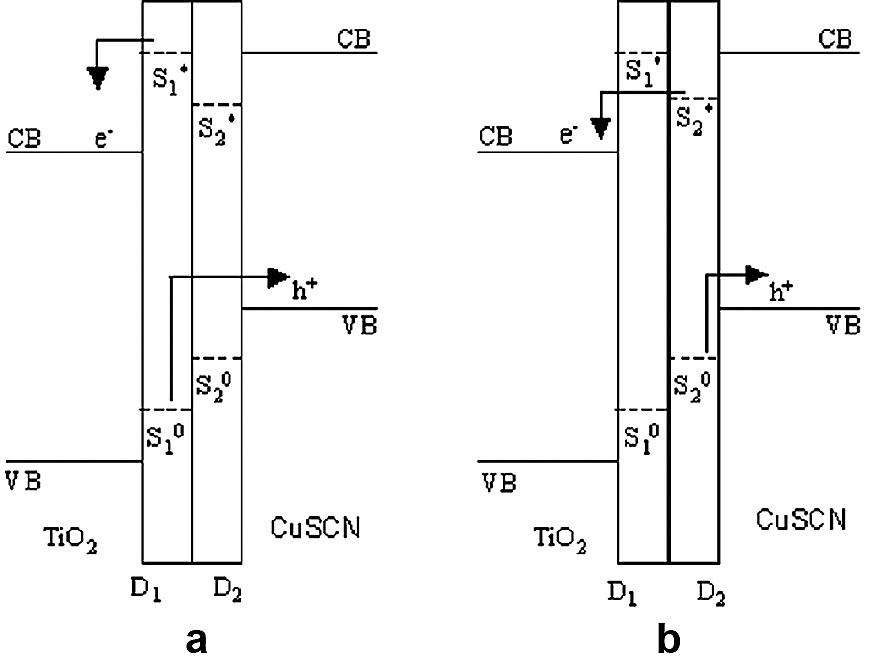
Schematic energy level diagram indicating the relative positions of conduction bands (CB) and valence bands (VB) of TiO2 and CuSCN and ground and excited levels of the D1 and D2: (a) charge transfer on excitation of D1, (b) charge transfer on excitation of D2.
If the electron and hole injection rates are faster than quenching, [i.e. processes (1)–(3)], the quantum efficiency of charge separation would be nearly unity.
The use of anionic and cationic dyes as described in the experimental section is a simple way coupling two dyes to form a structure of the configuration N/D1–D2/P as shown in Fig. 2c. Consider the sodium salt D1Na of an anionic chromophore D1. When the TiO2 film is exposed to a solution of D1Na, surface chelation would form a complex, which we represent by TiO2/D1Na. The subsequent treatment of the film with a solution of D2Cl (a chloride of the cationic chromopore) produces the structure TiO2/D1–D2 via the double decomposition reaction:
| (6) |
Figs. 4 and 5 indicate how MC, MV and BR, IR 786 gets attached to TiO2 surface by the above reaction. The mode of interaction of MV and IR 786 with CuSCN remain uncertain. Presumably, nitrogen sites in MV or IR 786 bonds to Cu atoms on the surface of CuSCN. The incident photons to photocurrent conversion efficiencies (IPCEs) of cells sensitized with MC, MV, MC-MV as reported earlier [8] and those of cells sensitized with BR, IR 786, BR-IR 786 are summarized in Tables 1 and 2. It is interesting to note that the IPCEs at the peak absorption wavelengths are higher for the double dye systems. Photocurrent action spectra of the cells sensitized with BR and BR- IR786 presented in Fig. 6 shows that in the latter system, sensitization is extended to near IR region. Table 3 summarizes the energy conversion efficiencies of cells sensitized with different dyes. The efficiencies of the double-dye cells are seen to be higher than those sensitized with individual dyes.
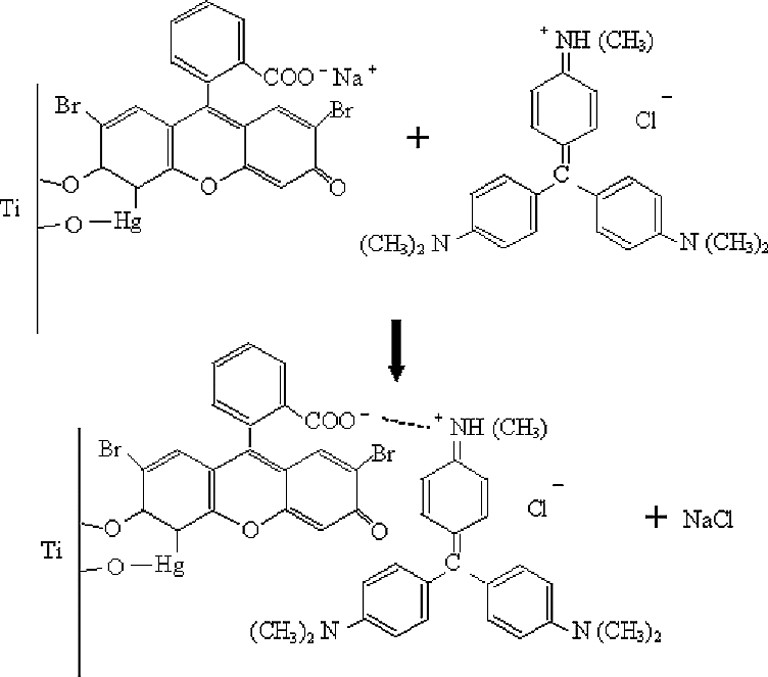
The mode of anchoring of mercurochrome to TiO2 and attachment of methyl violet cation by replacement of Na+.
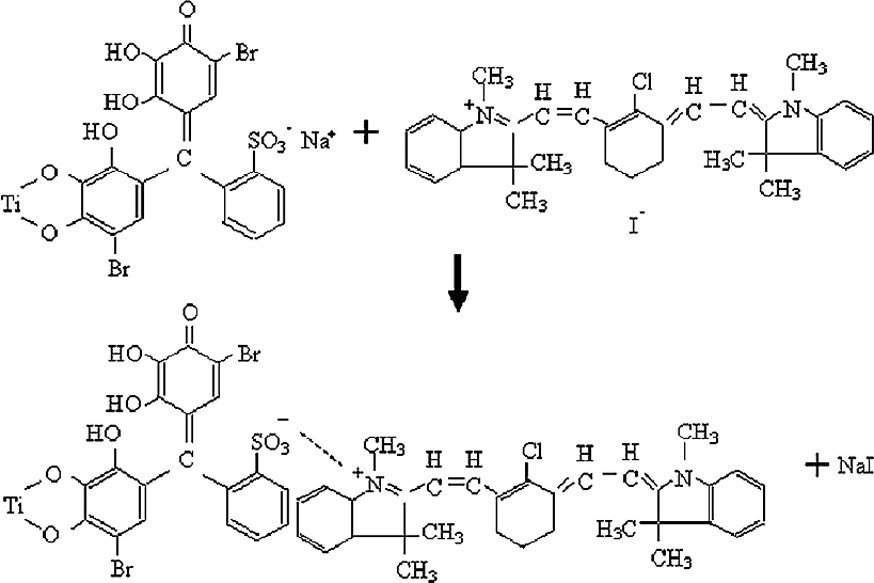
The mode of anchoring of bromopyrogallol red to TiO2 and attachment of IR 786 cation by replacement of Na+.
Incident photon to photocurrent conversion efficiencies (IPCEs) of the cells (1) TiO2/ MC-MV/ CuSCN, (2) TiO2/ MC/ CuSCN, (3) TiO2/ MV/ CuSCN at the peak absorption wavelengths of the two dyes
| Cell | IPCE% (λ = 550 nm) | IPCE% (λ = 620 nm) |
| MC-MV | 22.2 | 13.8 |
| MC | 15.2 | – |
| MV | – | 13.1 |
Incident photon to photocurrent conversion efficiencies (IPCEs) of the cells (1) TiO2/ BR-IR786/ CuSCN, (2) TiO2/ BR/ CuSCN, (3) TiO2/ IR786/ CuSCN at the peak absorption wavelengths of the two dyes
| Cell | IPCE% (λ = 590 nm) | IPCE% (λ = 800 nm) |
| BR- IR786 | 9.7 | 4.1 |
| BR | 6.9 | – |
| IR 786 | – | 1.0 |
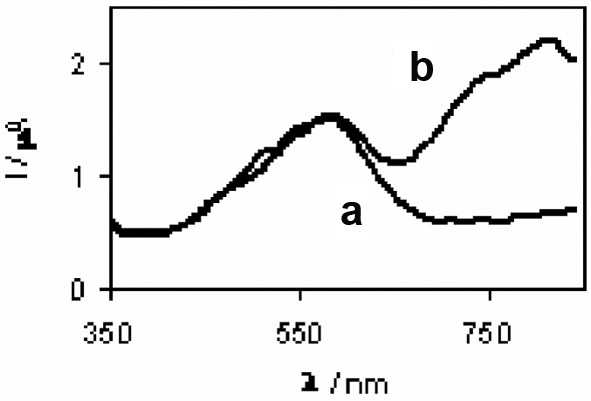
Photocurrent action spectrum of the cells (a) TiO2/BR/CuSCN, (b) TiO2/BR-IR786/CuSCN.
Open-circuit voltage (Voc), short-circuit photocurrent (Isc), fill factor (FF) and Energy conversion efficiency (η) of the cells: (1) TiO2/ MC-MV/CuSCN, (2) TiO2/MC/CuSCN, (3) TiO2/MV/CuSCN, (4) TiO2/ BR-IR786/CuSCN, (5) TiO2/BR/CuSCN, (6) TiO2/IR786/CuSCN
| Cell | Voc (mV) | Isc (mA cm−2) | FF (%) | η (%) |
| MC -MV | 629 | 4.6 | 47.3 | 1.37 |
| MC | 603 | 2.4 | 40.9 | 0.60 |
| MV | 407 | 1.3 | 38.5 | 0.21 |
| BR- IR 786 | 495 | < 3.2 | 41.5 | < 0.44 |
| BR | 494 | < 0.9 | 45.3 | < 0.14 |
| IR 786 | 420 | < 0.2 | 35.5 | < 0.02 |
Light-induced charge transfer in the systems that we have studied could involve intermediate steps. If we neglect extraneous interactions between dye molecules, the basic operating unit we need to consider is constituted of two chromophores (D1 and D2) linked by a bridge B and each chromopore bonded to n and p-type semiconductors on the opposite sides (Fig. 2c). The ionic nature of D1, D2 chromophores could result formation of a zwitterionic structure inducing a dipole electric field favoring charge transfer. Furthermore, these chromophores have donor- acceptor properties (e.g., anionic D1 and cationic D2 being the acceptor and donor respectively). Self-explanatory schematic diagrams indicating possible intermediate stages of charge-transfer schemes are presented in Fig. 7. Superexchange-type interactions and molecular rectification are likely advantageous features of double dye systems that may be exploited to broaden the spectral response and suppress recombinations. It is known that zwitterionic units of the structure E1/D–B–A/E1 (D = donor, A = acceptor, B = bridge, E1, E2 = external leads) have rectifying properties [11–14]. The dark rectification ratios for the cells MV, MC and MC-MV are found to be 12.6, 15.4 and 23.2, respectively and indicate that, as expected, the cell MC-MV has the best rectification property.

Schematic diagram indicating possible intermediate stages of charge injection to n-type (boxes on right) and p-type (boxes on left) semiconductors when dye molecules D1 (circles on left) and D2 (circles on right) are excited. (a) Excitation of D1 followed by electron transfer between two dye molecules and subsequent electron injection of n-type material and holes to the p-type material. (Similar steps occur when D2 gets excited). (b) Possible electron transfer schemes when carrier injection to the semiconductor is the initial step.
4 Conclusion
The above investigation suggests that coupling of two dye molecules to each other and to n- and p-type semiconductors would be a way of broadening the spectral response of dye-sensitized solar cells to enhance the efficiency. An additional advantage of this technique is suppression of recombination by molecular rectification. Models presented are suggestive of this effect. However, construction of practical systems based on this idea requires resolution of many technical problems. Methods will have to be found to avoid extraneous interactions between coupled molecules. If fast charge separation and good molecular rectification is achieved, quenching by these interactions will be less significant. Again more effective methods will have to be found to attach the dye D2 to the p-type semiconductor.


