1 Introduction
Polythiophene and its derivatives have been widely studied during the past years, due to their large application possibilities [1,2], including sensors and/or biosensors [3–7], electrochemical [8,9] and photovoltaic cells [10,11], energy storage [12–15], transistors [16–18], electroluminescent diodes [19], or protective coatings [20–22].
Among these compounds, polybithiophene (PBT) attracted considerable interest owing to its easier electrosynthesis: the oxidation potential of the monomer is lower than those of unsubstituted or 3-methyl-substituted thiophenes [1,2]. PBT has already been obtained on Pt in organic [23–25] and in micellar media [26,27], on Pt and oxidizable metals such as Fe, Al or Ti [22,28–30]. The use of micellar media (especially anionic ones) leads to more homogenous and regular structures [24–29].
The electrochemical characterization of PBT films in aqueous LiClO4 solutions showed dissymmetric oxidation and reduction peaks, describing the doping/undoping processes [23–30].
All these features explain our interest for studying the electrochemistry of PBT films by electrochemical impedance spectroscopy (EIS), which is a powerful tool to study charge transfer, ion diffusion, and capacitance of conducting polymer-modified electrodes [31]. EIS has been used to study polyoctylthiophene films in an organic medium [32,33], poly(3,4-ethylenedioxythiophene) [34–36], and polybithiophene films [37] in aqueous media.
In our previous works, we studied the electrosynthesis and electrochemical characterizations of poly(3-methoxythiophene) (PMOT) in aqueous micellar media (in the presence of different types of surfactants [38–40]). Very recently, we realized the EIS characterization of PMOT films electrosynthesized in an aqueous micellar medium (i.e. using sodium dodecylsulfate as surfactant) in aqueous LiClO4 solutions [41], and of polypyrrole in different types of electrolytes and solvents [42].
In this paper, we study by EIS the PBT films obtained in a micellar medium similar to that used for PMOT electrosynthesis. Lithium perchlorate was selected for these investigations because of the high doping properties of ClO4− anions for PBT films. The influence of the electrolyte concentration has already been studied and the optimal value is 0.3 M [41].
2 Experimental
2.1 Chemicals and apparatuses
The monomer (2–5 bithiophene (BT), 97%) and lithium perchlorate were obtained from Aldrich. Sodium dodecylsulfate (SDS) and n-butanol were purchased from Acros. All these reagents have been used as received.
The BT electropolymerization was carried out using an EG&G Princeton Applied Research Potentiostat/Galvanostat Model 283. This apparatus, coupled with a Schlumberger SI 1255 Frequency Response Analyzer, was also used in the EIS measurements.
2.2 Polymer electrodeposition
The electrosynthesis of polybithiophene (PBT) films was performed in a one-compartment cell, containing the working electrode (a Pt disc of 1-cm diameter), the saturated calomel electrode (SCE) as a reference electrode, and the counterelectrode (stainless steel wire). The electrolytic medium for electrosynthesis was prepared by dissolving 0.05 M BT + 0.1 M SDS + 0.1 M LiClO4 in a water–BuOH (90/10 v/v) mixture in an ultrasonic bath. The films were obtained by applying a constant current intensity (0.393 mA) during 100 or 200 s, corresponding to 50 and 100 mC/cm2. We assume that within this range, the film thickness varies linearly with the passing charge, like in the case of PMOT obtained under the same conditions [39]. The PBT electrosynthesized in this way is in its oxidized form and holds ClO4− and dodecylsulfate anions, as dopants (ClO4−-doping level: 15%, obtained by XPS analysis [26,27]). It was washed with water, dried with air and used like this in the impedance measurements.
2.3 EIS analysis
EIS measurements were carried out on the PBT films at various DC potentials (referred to hereafter as Edc) by applying 5 mV AC on the DC potential at frequencies ranging from 100 kHz to 0.01 Hz. The impedance data were fitted utilizing equivalent electrical circuits by using the ZSimpWin Electrochemical Impedance Spectroscopy (EIS) Data Analysis Software, delivered by EChem Software, USA. Because of a loss of accuracy at too high or too low frequencies, the simulations were limited to the range 10 kHz–0.1 Hz. The EIS measurements were performed at different applied DC potentials (0, 0.2, 0.4, 0.6 and 0.8 V/SCE). Before each EIS measurement, the electrode was held for an equilibrium time of 5 min. The EIS measurements were made in an aqueous LiClO4 0.3 M solution.
3 Results and discussion
3.1 Equivalent electrical circuit
The general aspect of the impedance and admittance curves of Pt/PBT electrodes is presented in Fig. 1. Two distinct parts can be noticed: a portion of semicircle in the region 10–10,000 Hz, and an oblique line between 0.1 and 4 Hz, which indicates presumably a diffusive capacitance.

Impedance curves of the Pt/PBT electrode (passing charge: 50 mC/cm2; Edc = 0.0 V/SCE). The calculated data, obtained by fitting of the experimental data to the model shown in Scheme 1, are: Q1 = 4.49 × 10−5 F/cm2 (n1 = 0.72), R2 = 949 Ω cm2, C3 = 6.68 × 10−6 F/cm2, R4 = 8.4 Ω cm2, Q5 = 1.28 × 10−4 F/cm2 (n = 0.82), R6 = 92.85 Ω cm2.
Several circuits were tested to fit the EIS spectra of PBT, including those found in the literature for other polythiophene derivatives [34–37], but were unsatisfactory (poor χ2 correlation coefficients). In contrast, we obtained good fittings for frequencies ranging from 0.1 to 10,000 Hz (χ2 ≈ 10−5–10−4) by using the equivalent circuit depicted in Scheme 1. The EIS spectra of PBT films were analyzed using the model (QR)((CR)(QR)). The circuit is similar to that of PMOT film impedance spectra, but the first pure capacitance observed in the case of PMOT [41] is replaced here by a constant-phase element with exponents close to 1. No solution resistance was found in the prospected domain, as for PMOT [41].
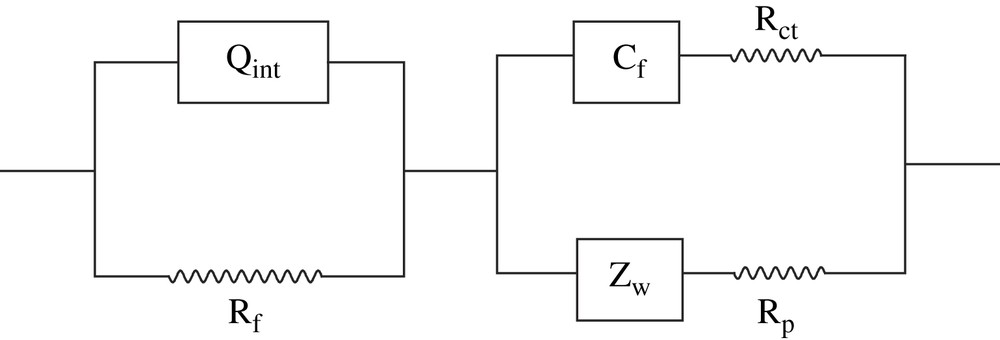
Equivalent electrical circuit for the Pt/PBT electrodes.
The first element (Qint) is assigned to an interfacial double-layer capacitance (the exponent values are 0.71–0.83) at the interface polymer|electrolyte and Rf is ascribed to the film resistance. Cf is the redox capacitance of the polymer itself, and is serially linked to the charge-transfer resistance (Rf). Rp is ascribed to a sum of resistances due to the electrolyte in the pores of the film and the 0.3 M LiClO4 solution. Finally, Zw is attributed to a diffusive capacitance (or Warburg impedance). It was fitted in the circuit as a constant-phase element (Q) which gave exponent values close to 0.5. This element describes the ions diffusion throughout the polymer film, essentially the release of perchlorate anions, and probably negligible amounts of dodecylsulfate anions. These latter species are difficult to remove from the film because of the high size of the surfactant hydrophobic chain.
3.2 EIS results
We performed EIS analysis of polybithiophene (PBT) films electrosynthesized in the aqueous micellar medium described above. The polymerization of BT in these conditions leads to a regular film of unsubstituted polythiophene [26,27]. In contrast to PMOT, the resulting polymer (PBT) is not soluble in the common solvents. This indicates a polymerization degree higher than that of PMOT.
3.2.1 Interfacial double-layer capacitance (Cint)
The electron transfer at the metal|polymer interface is generally considered as faster than the anion crossing through the polymer|electrolyte [37,45]. Qint occurs therefore most likely at the polymer|electrolyte interface. The values of the capacitance at the polymer|electrolyte interface (Qint) are comprised between 10 and 140 μF/cm2, with n values ranging from 0.71 to 0.83. The deviation towards a non-ideal capacitance behavior can be related to the PBT structure, formed of longer monomeric chains, and therefore different from PMOT one. For instance, exponent values comprised between 0.90 and 0.97 have been reported for a PBT film obtained in organic medium [37], indicating that our PBT film is more inhomogeneous, probably because of the simultaneous doping of DS− and ClO4− anions. As can be seen in Fig. 2, Qint decreases slightly with the film thickness, in contrast to what was observed for PMOT (increase of Qint when the film thickness increased [41]). However, it is important to note that our values are lower than those of PMOT previously obtained (1–2 mF/cm2) [41]. This evolution of Qint may be related to the fact that thin layers result usually in high capacitance, whereas thick layers yield small capacitance for ions situated at the surface of the layers. Our PBT films are presumably much thicker than PMOT ones, because of their easier electrosynthesis and higher electropolymerization yield. Qint seems to reach a maximum at the open-circuit potential, which was found to range from 0.65 to 0.69 V/SCE. This tendency (presence of a maximum) was observed with polypyrrole films [42–44], and was explained by the fact that the amount of quasi-free state ions reaches a maximum at the open-circuit potential.
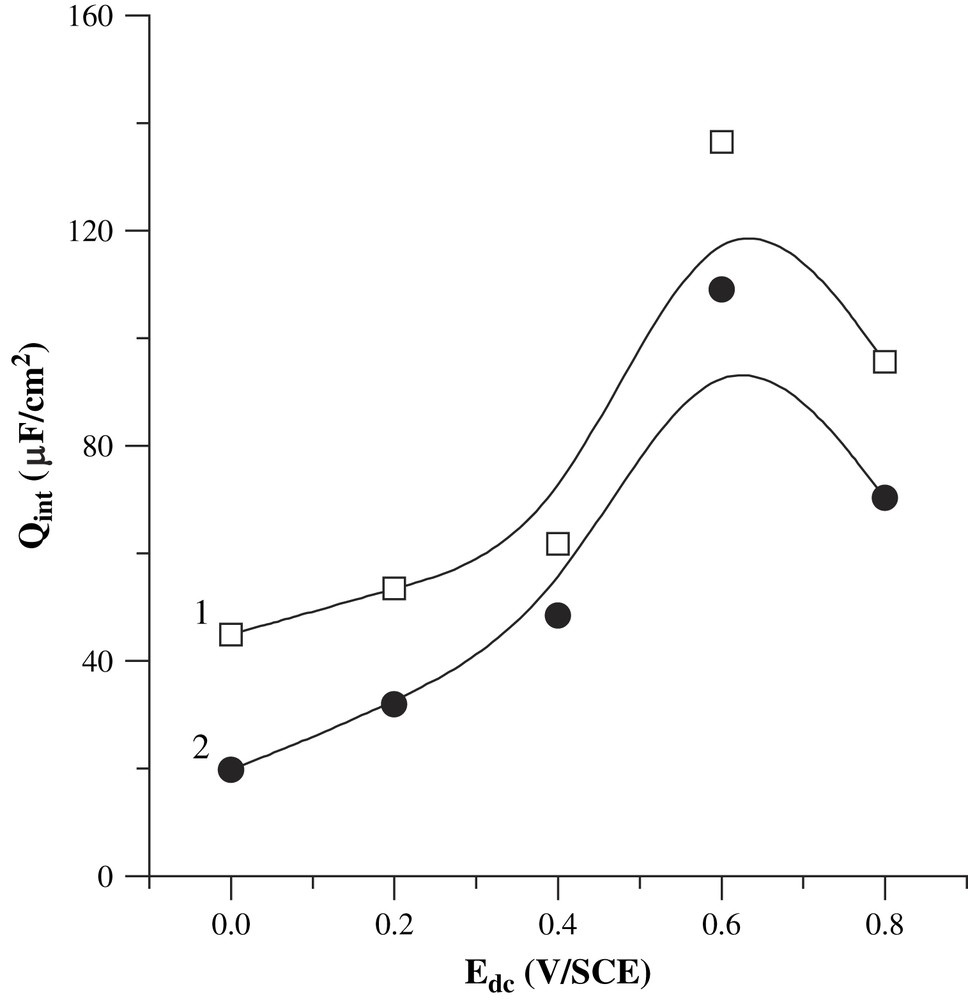
Evolution of Q1 with the applied potential, for the films obtained by applying 50 (1) and 100 mC/cm2 (2). The mean exponent values are, respectively, 0.74 and 0.80.
3.2.2 Film resistance (Rf)
The film resistance (Rf) was found to be comprised between 160 and 1000 Ω cm2. These values are higher than PMOT film resistances (4–15 Ω cm2 for the oxidized form [41]), and PBT (reduced form) in organic media (50–300 Ω cm2 have been reported for PBT films in acetonitrile, the thickness being comprised between 100 and 500 nm [37]). The high values of Rf suggest that after preparation, the PBT films are in a relatively low oxidized state. The variation of the film resistance with the applied potential is shown in Fig. 3. Rf decreases first with the applied potential, reaches a minimum at about 0.4 V/SCE, and then increases. This behavior was noted in the case of PMOT, as well as polymethylthiophene in aqueous LiClO4 solutions [45]. The minimum is not far from the open-circuit potential. However, after 0.7 V/SCE, we note an abrupt decrease in Rf because of a possible overoxidation of the polymer. The fact that Rf is higher for the thinner film can be related to an increase of the doping level of the polymer during its electrochemical growth process, as previously shown for unsubstituted polythiophene, doped with boron trifluoride diethyl etherate [46].

Variation of R2 with Edc for the two different PBT films, obtained with a charge of 50 (1) and 100 mC/cm2 (2).
3.2.3 Film capacitance (Cf)
The PBT film capacitance (Cf) is comprised between 2 and 30 μF/cm2 (Fig. 4). These results are much lower than those obtained for the PMOT oxidized form (0.4–5 mF/cm2), but are fairly higher than those of the PMOT neutral form (0.8–1.2 μF/cm2) [41]. Moreover, the values are comparable to those reported by other authors for PBT in organic medium (2–30 μF/cm2) [37]. In addition, Cf is higher for the thinner film. All these comparisons confirm that our PBT obtained in the micellar medium is not in the fully oxidized state. Fig. 4 shows that Cf increases slightly below the open-circuit-potential, but strongly after. As previously noted, the film was held for 5 min for equilibration. During this time, the doping level changes, and therefore, the augmentation of Cf with the potential can be related to the doping level of the film.
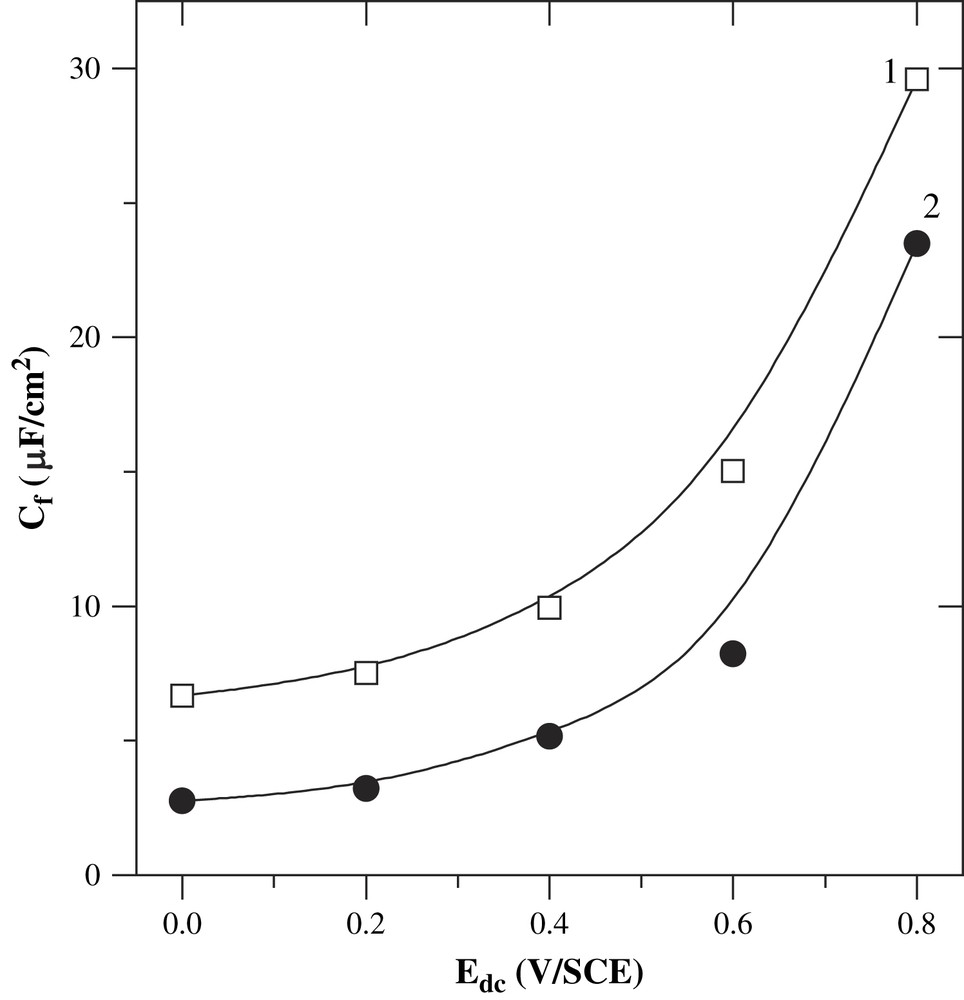
Evolution of C3 with the applied potential, at the two different passing charges during the electrodeposition of the PBT films, i.e. 50 (1) and 100 mC/cm2 (2).
3.2.4 Diffusive capacitance (Zw)
The evolution of the diffusive capacitance is shown in Fig. 5. Zw increases with the applied potential and with the passing charge as the ions are easier to remove from the thinner film. However, for relatively low applied potentials (0–0.2 V/SCE), the constant-phase exponent (n) is relatively high (0.77–0.80). This feature suggests that the overall doping reaction should not be absolutely diffusion-controlled. The diffusion of the ionic species and the chemical reaction rates may then be in the same order of magnitude when the applied potential is far from the open-circuit potential. The values of ZW for our PBT films (110–600 μF/cm2, with constant-phase exponent values (n) of 0.4–0.6) are comparable to those of PMOT films (Zw is comprised between 100 and 1300 μF/cm2 and n ≈ 0.6 for the PMOT oxidized form [41]). This result was predictable because these two oligothiophenes are doped by the same ions (perchlorate and dodecylsulfate anions). The evolution of the diffusive capacitance with the applied potential is shown in Fig. 5. Zw seems to decrease with Edc, but after 0.7 V/SCE, a probable overoxidation of the polymer gives unpredictable values of Zw.
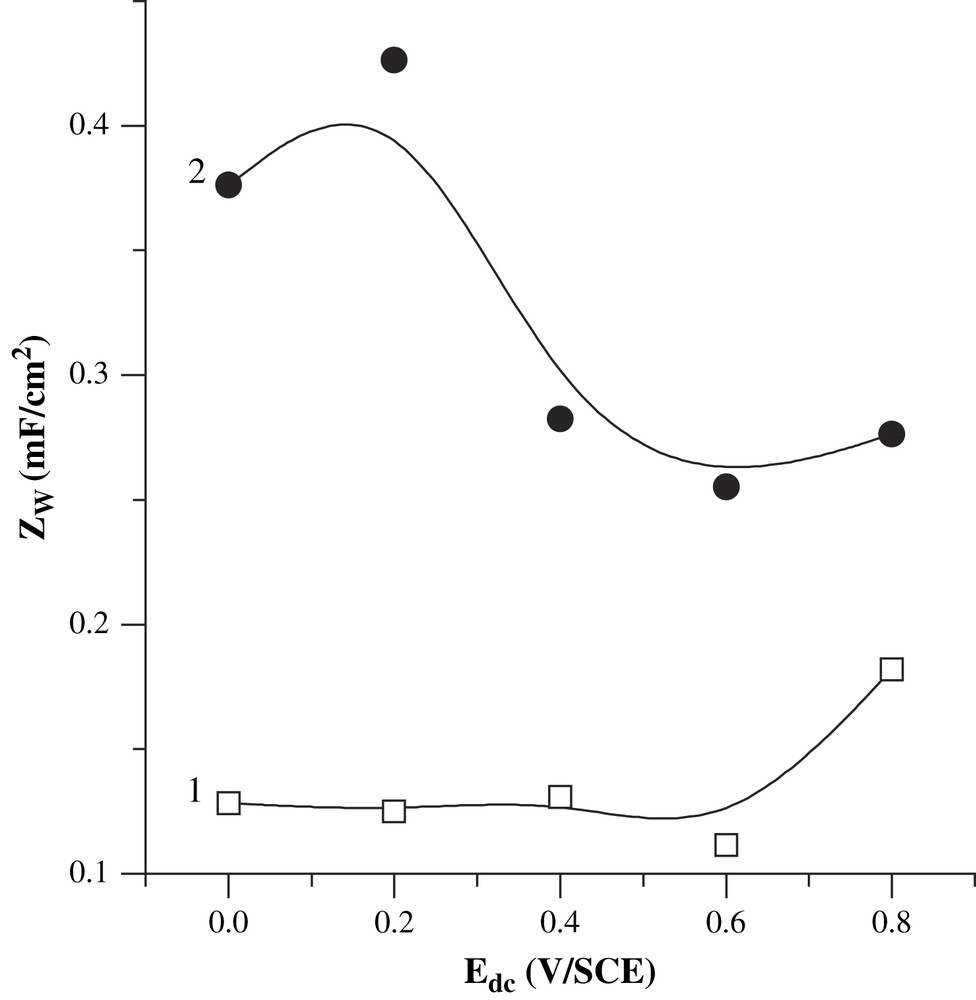
Evolution of Q5 with the applied potential, for the films obtained by applying 50 (1) and 100 mC/cm2 (2). The mean exponent values are, respectively, 0.41 and 0.64.
3.2.5 Charge-transfer resistance (Rct)
In the case of Rct, we obtain lower values (see Table 1) for PBT relative to PMOT (20–36 Ω cm2 [41]). Furthermore, they are confined in a more limited extent of values. In fact, Rct does not vary significantly with the applied potential and the film thickness. In contrast, our results are lower than PBT charge-transfer resistances obtained by other authors (Rct values of 50–300 Ω cm2 have been reported for the PBT reduced form [37]). There is likely a further doping during the EIS measurements on PBT obtained in the micellar medium.
Charge transfer and pore resistances at different applied potentials and passing charges
| Edc (V/SCE) | Rct (Ω cm2) | Rp (Ω cm2) | ||
| 50 mC/cm2 | 100 mC/cm2 | 50 mC/cm2 | 100 mC/cm2 | |
| 0 | 8.4 | 11.8 | 93 | 128 |
| 0.2 | 8.7 | 11.3 | 98 | 174 |
| 0.4 | 9.6 | 10.4 | 106 | 234 |
| 0.6 | 9.8 | 12.4 | 154 | 59 |
3.2.6 Pore resistance (Rp)
Finally, the variation of the pore resistance (Rp) with the film thickness is shown in Table 1. Rp, which is assigned to a sum of resistances due to the electrolyte in the pores of the film and the 0.3 M LiClO4 solution, increases with the film thickness, on the contrary to PMOT films, for which Rp decreased with the film thickness [41]. This behavior of Rp may be related to the porosity extent of the PBT, which is higher, in the thicker film. As for PMOT, Rp was found to increase with the applied potential (30–70 Ω cm2 for a PMOT film of 0.375 μm thickness), but the reason is not actually clear to us. Such a resistance was also found in the equivalent-electrical circuit of polythiophene-coated steel, studied in NaCl aqueous solution, but was not quantified [47]. The presence of a pore resistance suggests that the film should be highly porous and contain an excess of electrolyte, which facilitates anion diffusion.
4 Conclusions
In this work, we realized the EIS characterization of PBT films on Pt and in an aqueous 0.3 M LiClO4 solution. We proposed an equivalent electrical circuit with an interfacial double-layer capacitance, a diffusive capacitance due to the diffusion of the dopants in the polymer and a third one (Cf, describing the redox properties of the film), associated with three different resistances. All of them are affected by the film thickness and the applied potential. The EIS measurements are continuing on other conducting polymers, other solvents, and other electrolytes. We are also presently performing contact angle measurements on a series of conducting polymers in order to complete the characterization of this class of materials.
Acknowledgements
This work was partly supported by the Third Word Academy of Sciences (TWAS) throughout by a project grant to the Senegalese Research Group in Electrochemistry and Polymer Science (TWAS Research Unit No 04-050 LDC/CHE/AF/AC).


