1 Introduction
Meat stock, i.e. the aqueous solution obtained by thermal processing of animal tissues in water, has been a very important food in history of mankind, as it is already mentioned by Apicius, four centuries BC [1]; in the 17th, 18th and 19th centuries, stock recipes were put at the beginning of most French culinary books [2–4]. Since the 18th century, many chemists were interested in meat-stock preparation [5–9]. Antoine-Laurent de Lavoisier (Paris, 1743—id., 1794) is perhaps the most famous chemist involved in this work. In 1783, he studied meat-stock preparation using density to evaluate its quality [10]. We report here experimental tests of his results.
Lavoisier was a ‘general farmer’ as well as a member of the Academy of Sciences, to which the government asked questions of social or political interest that needed experiments [11]. He was interested in meat stock after the Minister of Navy consulted the Royal Society of Medicine about the diet that hospitals should use. It was recognized that meat stock was not properly known, and, in particular, that the proportions of meat and water and the quality of meat used were not assessed. In his 1783 article, Lavoisier quotes Geoffroy le Cadet [8], who published in 1730 a work on the same topic: Geoffroy le Cadet wanted to analyze the chemical composition of stock, so that he boiled meat many times, always changing the water he used (he later evaporated the water to know the quantity of ‘extractive’ material dissolved from the meat). In comparison, Lavoisier wanted only to know the total mass of the ‘matter’ (he writes “gelatinous matter”) that meat was loosing in water during stock preparation done according to traditional culinary rules. It should be noted that Lavoisier, as other chemists of the same time, assumes that the dry matter is “nutritious”, not considering the chemical composition of this dry matter. This assumption was the basis of a later dispute between French and German chemists [12].
There is no ‘Materials and Methods’ section in Lavoisier's paper. However, he describes carefully how he determined dry matter of stocks using a special densitometer he built himself (see below). Using this device, Lavoisier wrote that he could determine density with a precision of 10−6, but he did not explain how he was doing this calculation. His first study was to determine the proportion of meat and stock that makes a ‘proper’ stock. He changed these proportions from four pounds of water per pound of meat up to one pound of meat for one pound of water. His results for Semimembranosus beef muscle are given in Table 1 and plotted in Fig. 1; the perfect correlation (R2 = 1) between density and dry matter of stocks at different proportions of meat is surprising.
Density and dry matter results from Lavoisier
| Proportion of meat/water | Density measured (data from Lavoisier) | Dry matter (g for 1 l of water added to the meat) | Sensory appreciation |
| 0.25 | 1.002322 | 3.8871 | Very weak |
| 0.5 | 1.003080 | 5.1907 | Weak but enough for convalescent |
| 1 | 1.007347 | 12.6763 | Strong, good |
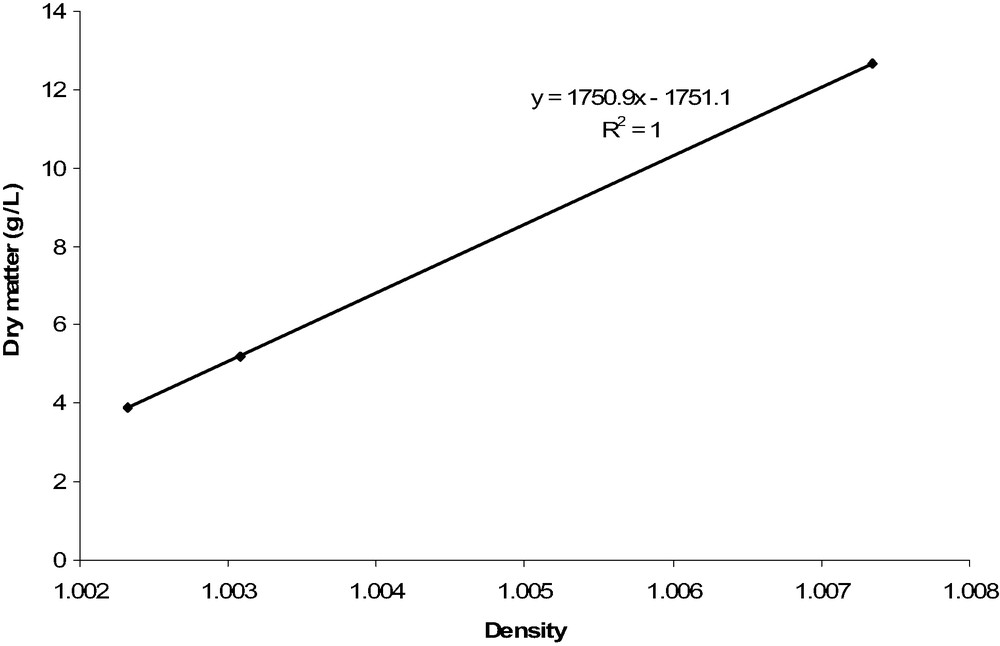
Plot of the dry matter as a function to density, as determined by Lavoisier.
Anyway Lavoisier concludes from his experiments that “the proper proportion to make stock is about two part of water for one part of meat.” This conclusion is based on a very rough interpolation of a limited number of experiments (it is not even said if the experiments were repeated with different meats from one animal or of different animals). A second conclusion given in the paper is that there is a good correlation between density and dry matter content. Third Lavoisier concludes that there is more extracted matter when meat is boiled in a large quantity of water than when boiled in a limited quantity of water. Then Lavoisier compares different kinds of meat of the same animal and observes large differences in density and in dry matter. In this part, it is said that cooking lasted 7–8 h, in boiling water. Again, the same correspondence appears between density and dry matter content, which makes Lavoisier concludes that a physician “who wants to know how much nutritious matter he gives to some customer can measure the density of stock.”
What can we keep today from such studies? We repeated Lavoisier's experiment in much more controlled conditions, compared them with results from different methods.
2 Materials and methods
In our experiments, we used the same ‘culinary’ method as Lavoisier, but we followed a much stricter protocol. In particular, meat was heated in distilled water, in vessels equipped with reflux column, so that no ‘matter’ could escape and a better appreciation of what was lost from the meat could be measured. As Lavoisier gave no indication on the quantity of sodium chloride he used, we used no such salt.
Meat and stock were weighed at various times during cooking with a Mettler PJ300 balance of precision 0.001 g and a Mettler H15 balance of precision 0.0001 g.
Beef meat was purchased from the local butcher (Boucherie de la Montagne, Paris). In all experiments, one single piece was divided into two parts, so that about the same quantity of fat and collagen tissue was present in both pieces (these quantities were not assessed). One piece was processed at T = 100 °C with a large quantity of water (details given below), and the other with a smaller quantity of water (same temperature). In order to see potential differences, we increased the difference of water content in both cases.
2.1 Series of experiments No. 1
(a) Meat pieces of two origins were used: (I and II) Supraspinatus; origin France; producer SARL Chardon, 42310 Chagny; female having bred, No. 206675 Charolais Terroir; slaughtering No. 68922); (III and IV) Semimembranosus; origin France, Limousine, no other indication). For these pieces, slices (thickness about 1 cm) were cut perpendicularly to the fibers and cooked either with a small or a large quantity of water, until the meat disrupted (885 min for I and II, 1268 min for III and IV).
(b) Semimembranosus (origin France; producer Louis Cannet, 71140 Chateauneuf; female that did not bred; animal number 112; weight 245.900 kg; number 68729; birth date 27/03/1999; slaughtering No. EEC: 01.187.01) was divided into four parts:
- ● (I) 48.39 g;
- ● (II) 70.38 g;
- ● (III) 80.67 g;
- ● (IV) 73.33 g.
Meat pieces I and II were put in 215 ml water, whereas pieces III and IV were cooked with 3200 ml water. At various times, the pieces were taken out of the heating vessel, dried using a cloth and weighed.
2.2 Series of experiments No. 2
Here Biceps femoris (same origin than in experiment 1b) was used to determine the dry matter content of stock prepared either with a large or a small quantity of water. Samples of stock were taken up using a precision syringe (the quantity was weighted using the balance), and dried under vacuum for 2 days.
In order to keep the water volume constant, a quantity of water equal to the quantity of stock taken up was added.
In order to test the importance of gelatine present in the solution, we compared meat (Biceps femoris, same origin than in experiment 1b) pieces of precisely know mass (about 200 g) cooked in the same quantity of water (500 g), but with a different quantity (precisely weighted, respectively, about 10 and 40 g) of dissolved gelatine (gelatine sheets for food uses, quality brand No. 1, Elodie, Bressols Inc.).
3 Results and discussion
3.1 Series of experiments No. 1: total losses
In order to make a balance between what goes out from the meat, and what comes into the stock, we began our experiments by measuring the total losses from meat (a). It was measured that water content in the vessel increased during cooking, because of meat contraction (this contraction is due to collagen denaturation). However, the method used is not precise enough to observe the effect described by Lavoisier, in particular because there are large differences according to the different pieces of meat (Lavoisier gave no information on how to take this phenomenon into account and even if he considered it). Accordingly, we changed the method and made a kinetic study (b). Here again, no significant difference could be measured using this protocol, because of the large uncertainties on meat masses (Fig. 2).
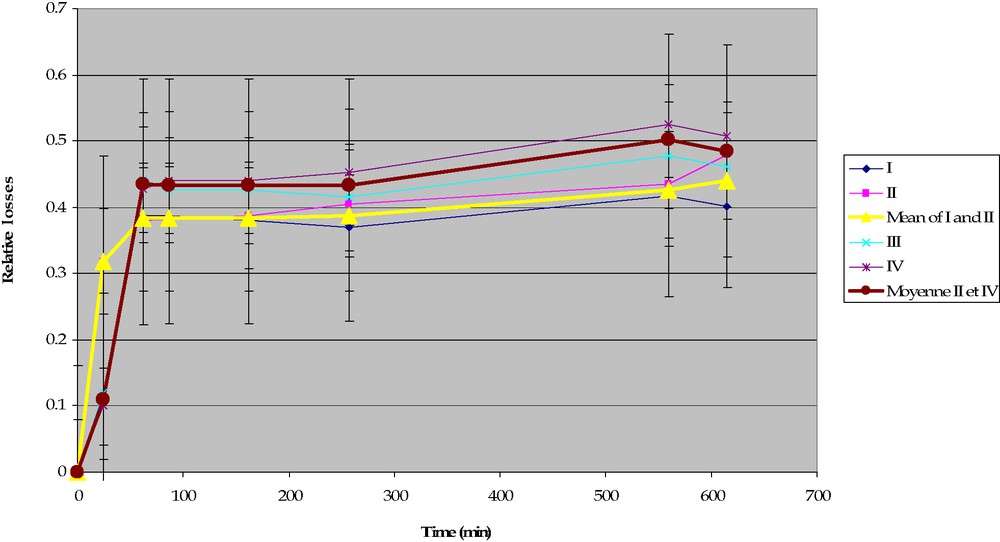
Measurement of dry matter in meat stock, series no. 1.
3.2 Series of experiments No. 2: kinetic of dry matter losses
In order to improve the results, we moved to measurements of the dry matter content of stock, as Lavoisier did. Results are given in Table 2. In order to calculate the dry matter quantity recovered from meat, assumptions were based on other experiments [12] that showed that, in the same conditions as in the experiments done for this study, the mass of the meat is decreasing for about 2 h, after which the decrease is very slow, as shown in Fig. 3. As the first sample of experiment No. 2 is obtained after more than 2 h, the dry matter content is calculated on the basis of the final volume (335.501 g for I and 1091.857 g for II). This dry matter content is given in Fig. 4.
Total losses in experiment No. 2
| I | II | |
| Initial mass (g) | 147.930 | 156.050 |
| Water mass (g) | 250.000 | 1000.000 |
| Total mass in the vessels | 397.93 | 1156.05 |
| Final meat mass (g) | 62.429 | 64.193 |
| Mass lost by the meat (g) | 85.501 | 91.857 |
| Relative loss | 0.58 | 0.59 |
| Final mass of water (g) | 335.501 | 1091.857 |
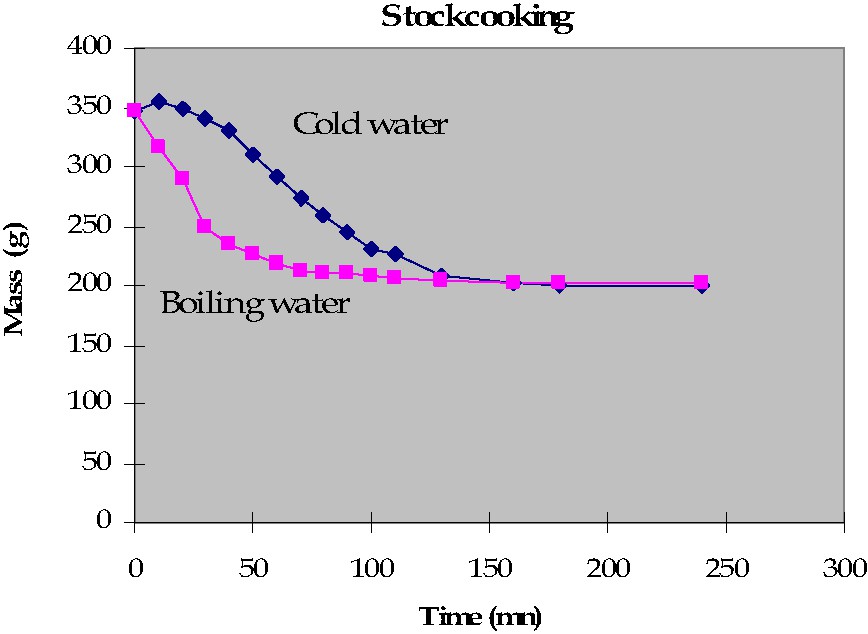
Mass of meat cooked in function of time.
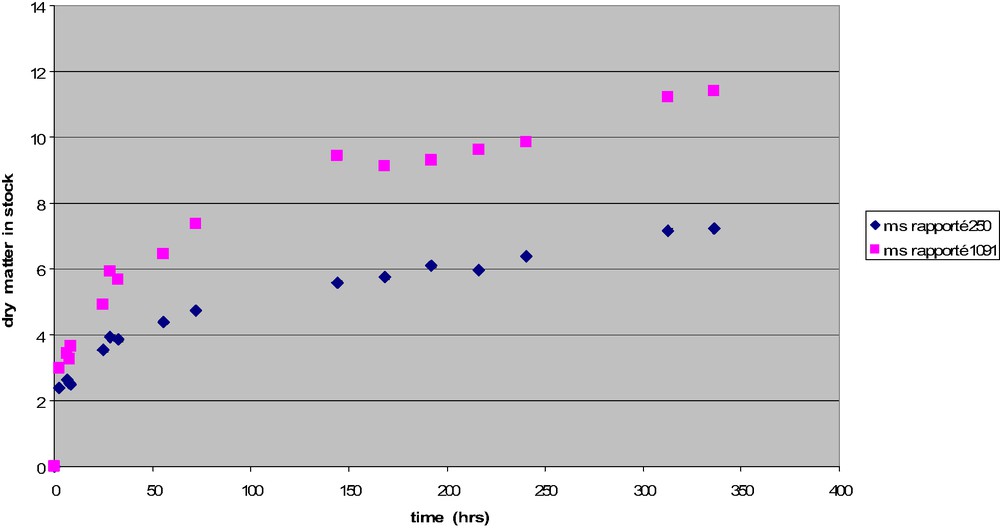
Dry matter in meat stock in function of time for two meat/water proportions.
Strictly speaking, our results cannot be compared to Lavoisier's, because he did not indicate how long he was cooking the meat, but we find the same overall tendency: meat stocks obtained from a certain quantity of meat have more dry matter content when the volume of the solution is larger. Why? This is not obvious, and should be explained using a first-order model of meat [13]. Muscles are two-part compartments system. Muscular fibers, on the one hand, are limited by membranes made of phospholipids (with protein channels that can transport specific ions). These cells are covered by collagen tissue that makes bundles, and bundles of bundles, etc. Accordingly, exchange with the solution should be limited. Indeed previous experiments showed that the mass of meat cooked in water is not changing much with time, after about 2 h of process, when the temperature inside meat is equal to the temperature in stock; during a first step, before this time, collagen shrinking is probably controlling water losses from the meat toward the stock [14]. Later, dry matter increases because of collagen gelatinization, collagen diffusion and collagen hydrolysis. This can explain why dry matter increases with time. During this second step, diffusion from the inside of muscular fibers, or within the gelatinized collagenic tissue, is dependent on some driving force, which is the concentration difference between the gelatinized tissue and the solution. In order to test this assumption, we cooked two pieces of meat in the same conditions, except that the cooking medium of one piece was added with pure gelatine: the significantly different losses (respectively, 47 and 51%) could explain the differences in dry matter content in stocks made using little or much water.
3.3 Series of experiment no. 3: density measurements
Even if Lavoisier was right in assessing the dry matter content of stock, the R2 = 1.0 regression coefficient that can be calculated from his data is surprising. Moreover, the six digits on density values are notoriously difficult to measure. This is why we repeated his protocol in order to check his results.
Lavoisier described carefully the densitometer he used: “Le pèse liqueur dont je me sers est un cylindre creux formé d'une feuille d'argent mince, assez forte cependant pour ne pas se plier et se déformer quand on essuie l'instrument. Ce cylindre est lesté par le bas avec de l'étain fin, et il est surmonté, à son extrémité supérieure, par une tige de fil d'argent de 3/4 de ligne environ de diamètre, à laquelle est adapté un petit godet destiné à recevoir des poids ; j'ai fait une marque sur la tige à l'endroit jusques auquel le pèse-liqueur doit être enfoncé. Lorsque cet instrument est construit et qu'il est lesté de manière à être un peu plus léger que le volume d'eau qu'il déplace, on le pèse à une balance très exacte, on le plonge dans de l'eau distillée, puis on ajoute, sur le petit bassin supérieur, le nombre de grains et de fractions de grain nécessaire pour le faire enfoncer jusqu'à la marque pratiquée sur la tige ; on fait la même opération avec la liqueur dont on veut déterminer la pesanteur spécifique, et par le rapport du poids total, tant du pèse-liqueur que des grains qui y ont été ajoutés, on conclut la pesanteur spécifique en millionièmes. Le pèse-liqueur que j'emploie déplace un peu plus de 9 onces d'eau distillée. Je suis entré dans quelques détails sur cet instrument, parce qu'il peut être d'un usage commode dans un grand nombre d'opérations de pharmacie, et que, d'ailleurs, quoique je m'en serve habituellement, je n'en ai donné la description dans aucun autre mémoire.” [Personal translation: “The densitometer that I used is a hollow cylinder made from a silver sheet, thick enough to avoid bending and deforming while the tool is whipped. This cylinder is added with weights at the lower tip with tin, and its upper part is adjusted with a silver thread about 3/4 of line, the upper tip being fitted with a small bowl that can accept weights; I made a mark on this rod at the level to which the densitometer had to reach. When this tool is constructed and when the weights are fitted so that it is slightly lighter than the water volume it displaces, it is weighted with a very precise balance, it is put in distilled water, then in the upper bowl small weights are added so that the mark on the rod is reached; then the same operation is done with the liquid whose density is to be measured, and the density in millionth is calculated. The densitometer that I used dislodges slightly more than 9 onces of distilled water. I gave some details on this tool because it can be used in many experiments of drug confectionary, and, as I use it in other memoirs, I did not give indications to make it elsewhere].
Repeating Lavoisier's experiment showed how careful an experimentalist he was. In particular, the idea of having a very thin tube at the upper part is clever, because it is easily calculated that between two rods of radius R1 and R2, the ratio of sinking is (R2/R1)2.
In our experiments, we replaced Lavoisier's silver cylinder with a capillary tube (Hirschmann Laborgeräte 5 μl, 7.642 cm long, external diameter 1.3 mm, R ≤ 0.3%; or Corning capillary tube 20 μl). This tube was glued on top of a fishing float with lead beads at the bottom, so that its sinking could be precisely adjusted, as Lavoisier did. As in Lavoisier's experiments, a bowl was glued at the top of the capillary tube, and small pieces of paper were added so that the rod sank to a certain level written on the rod. Distilled water was compared to a solution made by dissolving a precisely weighted quantity of previously dried gelatine; all our experiments were done at a carefully controlled temperature (± 0.1 °C), in order to avoid density variations with temperature (Lavoisier did not give any indication that he checked temperatures).
Calculating the density d of the solution of gelatine is obtained by solving a two-equation system whose solution is: d = 1 + (m/M), where m is the mass added in the upper bowl and M the mass of the densitometer (the calculation shows how smart it was to keep the densitometer at the same level, in terms of uncertainties). With the values we used, the density was found equal to 1.0018. Unless Lavoisier did use some device that was not described in his article, it is doubtful that he could obtain a precision better than ours (0.0001). But it is also clear, from our experiments, that he was able to see the differences in stocks. The question of the R2 = 1 remains.


