1 Introduction
1,3-Oxazolidin-2-ones are structural components of many compounds that display pharmacological properties [1–11]. Furthermore, they have also been used as chiral auxiliaries and intermediates in asymmetric synthesis of numerous pharmaceutical products [12,13].
During the past few years, significant progress has been made in the discovery of new biologically active N-aryl and N-alkyloxazolidin-2-ones [14–16]. However, the N-sulfonylated oxazolidin-2-ones were not sufficiently elaborated, and only few reports have described the synthesis of N-tosyloxazolidin-2-ones [17–19].
Several methods have been employed for the synthesis of racemic and optically active oxazolidin-2-ones. One of the most efficient methods to build the heterocyclic carbamate involves the condensation of 1,2-amino alcohols with carbonyl derivatives such as phosgene [20], trichloromethyl chloroformate [18], bis-(trichloromethyl) carbonate [21], isocyanates [12], chloroformates [22], ureas [23], or diethylcarbonate [24]. The catalyzed addition of CO2 to aziridines has also been employed to prepare racemic N-tosyloxazolidin-2-ones [17].
Izuhara et al. [18] have described a four-step synthesis of the optically pure 4-benzyl-3-tosyloxazolidin-2-one starting from (l)-phenylalanine. In this sequence, the trichloromethyl chloroformate has been employed as a carbonylating agent. In this work, we describe a three-step synthesis of a variety of new chiral N-arylsulfonyloxazolidin-2-ones, using the same strategy cited above. We have employed the bis-(trichloromethyl) carbonate (BTC) instead of the trichloromethyl chloroformate, since it appears to be safer due to its lower vapor pressure and higher stability [25].
2 Results and discussion
Enantiomerically pure N-arylsulfonyloxazolidin-2-ones are prepared in three steps from commercially available (d)- and (l)-amino acid. As illustrated in Scheme 1, sulfonylation of α-amino acids with arylsulfonylchlorides, in a two-phase mixture of i-PrNEt2 in acetone and aqueous NaOH, leads to the N-arylsulfonyl amino acids 1a–p, which were then reduced to the N-arylsulfonyl amino alcohols 2a–p with good to excellent yields (Table 1) by use of lithium aluminium hydride in THF or (THF:Et2O). The same procedure has been employed by Berry and Craig [26] to prepare N-tosyl-α-amino alcohols from α-amino acids. These authors have determined the enantiomeric purity of N-tosyl-α-amino alcohols (entry 2m–p) by the formation of the corresponding MTPA esters [27], the minor diastereoisomers have not been detected in the 500 MHz 1H NMR spectrum of the crude product [26].
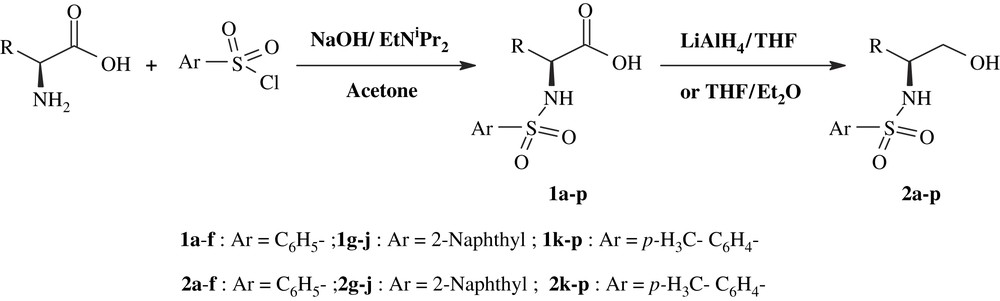
Synthesis of N-arylsulfonyl-α-amino alcohols.
Preparation of N-arylsulfonyl-α-amino alcohols
| Entry | R | Ar | Config. | [α]D | m.p. (°C) | Yields (%) |
| 2a | i-Pr | C6H5– | S | −10 | 76–78 | 98 |
| 2b | Me | C6H5– | S | +15 | Oil | 97 |
| 2c | i-Bu | C6H5– | S | +18 | 102–104 | 99 |
| 2d | Ph | C6H5– | R | −10 | 114–116 | 98 |
| 2e | Bn | C6H5– | S | +18.7 | 64–66 | 95 |
| 2f | s-Bu | C6H5– | S | +25 | 58–60 | 99 |
| 2g | Me | 2-Naphthyl | S | +23 | 82–84 | 82 |
| 2h | i-Pr | 2-Naphthyl | S | −10 | 96–98 | 86 |
| 2i | s-Bu | 2-Naphthyl | S | +18 | 112–114 | 85 |
| 2j | Bn | 2-Naphthyl | S | +22 | 106–108 | 75 |
| 2k | s-Bu | p-H3C–C6H4– | S | −15 | 80–81 | 98 |
| 2l | Ph | p-H3C–C6H4– | R | −10 | 93–94 | 97 |
| 2m | i-Pr | p-H3C–C6H4– | S | +16.8 | 88–89 | 98 (99)a |
| 2n | i-Bu | p-H3C–C6H4– | S | +23.7 | 105–106 | 96 (98)a |
| 2o | Bn | p-H3C–C6H4– | S | −15 | 74–75 | 97 (99)a |
| 2p | Me | p-H3C–C6H4– | S | +15.8 | 57–58 | 98 (100)a |
a Chemical yields 2m–p reported by Berry and Craig [26].
As far as we know, compounds 2a–l were prepared for the first time during this study. Compounds 2m–p were prepared by Barry and Craig [26].
Subsequent reaction of compounds 2a–p with BTC, in the presence of Et3N at −78 °C to r.t., provided the corresponding N-arylsulfonyloxazolidin-2-ones 3a–p in excellent yields without racemization, as confirmed by chiral HPLC analysis on tow compounds (entries 3c and 3h) (Scheme 2).
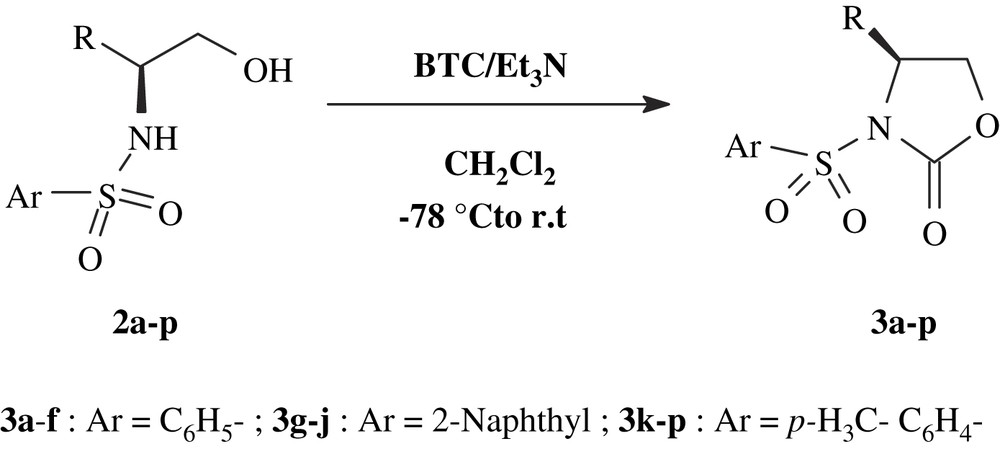
Synthesis of N-arylsulfonyloxazolidin-2-ones.
In Table 2, are given the chemical yields and the physical properties of compounds 3a–p, which have not been reported to date, except compound 3p.
Preparation of N-arylsulfonyloxazolidin-2-ones
| Entry | R | Ar | Config. | m.p. (°C) | [α]D | Yields (%) |
| 3a | Bn | C6H5− | S | 107–109 | +38.3 | 96 |
| 3b | i-Pr | C6H5– | S | 99–101 | +56.8 | 95 |
| 3c | Me | C6H5– | S | 60–62 | +45.0 | 95 |
| 3d | i-Bu | C6H5– | S | 139–141 | +39.6 | 96 |
| 3e | Ph | C6H5– | R | 118–120 | −13.6 | 97 |
| 3f | s-Bu | C6H5– | S | 144–146 | +60.0 | 95 |
| 3g | Me | 2-Naphthyl | S | 129–131 | +40.1 | 88 |
| 3h | i-Pr | 2-Naphthyl | S | 141–143 | +16.7 | 85 |
| 3i | s-Bu | 2-Naphthyl | S | 130–132 | +60.0 | 81 |
| 3j | Bn | 2-Naphthyl | S | 126–128 | +71.4 | 79 |
| 3k | i-Pr | p-H3C–C6H4– | S | 115–118 | +58.2 | 95 |
| 3l | i-Bu | p-H3C–C6H4– | S | 151–153 | +38.4 | 96 |
| 3m | s-Bu | p-H3C–C6H4– | S | 170–172 | +41.0 | 95 |
| 3n | Me | p-H3C–C6H4– | S | 117–119 | +52.2 | 97 |
| 3o | Ph | p-H3C–C6H4– | R | 149–151 | −39.5 | 95 |
| 3p | Bn | p-H3C–C6H4– | S | 136–138 | +36.2 | 96 |
3 Conclusion
We have synthesized, in good yields, a variety of new chiral N-arylsulfonyloxazolidin-2-ones from their corresponding α-amino acids. The test of the biological activity and the study of synthetic properties of these new products are under investigation in our laboratory.
4 Experimental section
TLC was performed on Merck 60F-254 silica gel plates (layer thickness 0.25 mm). Column chromatography was performed on silica gel (70–230 mesh) using ethylacetate and cyclohexane mixture as eluents. Melting points were determined on a Electrothermal 9002 apparatus and are uncorrected. 1H NMR spectra were recorded at 300 MHz. All chemical shifts are reported as δ values (ppm) relative to internal tetramethylsilane. CH2Cl2, THF were respectively distilled over CaH2 and Na/benzophenone. Elemental analyses were carried out by ‘Service de microanalyse’ of ‘Institut national de recherche et d'analyse physico-chimique de Tunis’. HPLC analyses were conducted on a methanol/hexane [70:30] system with a UV detector at 254 nm, using a Chirobiotic V column (250 × 46 mm) and a flow rate of 0.6 mL/min.
N-Arylsulfonyl amino alcohols 2a–p were prepared according to literature [26]; compounds 2m–p were reported by Berry and Craig [26].
4.1 (2S)-N-(Phenylsulfonyl)valinol: 2a
Yield = 98%; m.p.: 76–78 °C [hexane:ethylacetate (90:10)]. IR (cm−1): νNH = 3215, νOH = 3493; [α]D = −10 (c = 1, CHCl3). 1H NMR (300 MHz, CDCl3): 0.70–0.79 (2d, 6H); 1.73–1.82 (m, 1H); 2.22 (s, 1H); 3.03–3.09 (m, 1H); 3.54–361 (m, 2H); 5.24 (d, 1H); 7.28–7.60 (m, 3H); 7.92 (d, 1H). 13C NMR (75 MHz, CDCl3): (18.80, 19.49, 2CH3–); (29.83, –CH–); (61.48, CH–NH–); (63.41, –CH2–O–); (127.49–140.93, Carom). Anal. calc. for C11H17NO3S (243.32): C, 54.30; H, 7.04; N, 5.76. Found: C, 54.20; H, 7.20; N, 5.72.
4.2 (2S)-N-(Phenylsulfonyl)alaninol: 2b
Yield = 97%; oil; [α]D = +15 (c = 0.6, CHCl3). IR (cm−1): νNH = 3217, νOH = 3477. 1H NMR (300 MHz, CDCl3): 0.98 (d, 3H); 3.01–3.06 (m, 1H); 3.08 (s, 1H); 3.37–3.58 (m, 3H); 5.75 (s, 1H); 7.48–7.60 (m, 3H); 7.91 (d, 2H). 13C NMR (75 MHz, CDCl3): (17.28, 1CH3–); (51.91, –CH–NH–); (66.47, –CH2–O–); (127.35 and 141.01, Carom). Anal. calc. for C9H13NO3S (215.27): C, 50.22; H, 6.09; N, 6.51. Found: C, 50.10; H, 6.10; N, 6.42.
4.3 (2S)-N-(Phenylsulfonyl)leucinol: 2c
Yield = 99%; m.p.: 102–104 °C [hexane:ethylacetate (90:10)]; [α]D = +18 (c = 1, CHCl3). IR (cm−1): νNH = 3201, νOH = 3397. 1H NMR (300 MHz, CDCl3): 0.61–0.68 (2d, 6H); 1.22–1.51 (m, 2H); 3.02 (s, 1H); 3.17–3.56 (m, 3H); 5.25 (d, 1H); 7.30–7.53 (m, 3H); 7.86 (d, 2H). 13C NMR (75 MHz, CDCl3): (20.24, 22.30, 2CH3–); (38.75, –CH2–); (51.72, CH–NH–); (64.92, –CH2–O–); (126.48–140.25, Carom). Anal. calc. for C12H19NO3S (257.35): C, 56.01; H, 7.44; N, 5.44. Found: C, 55.80; H, 7.42; N, 5.39.
4.4 (2S)-N-(Phenylsulfonyl)phenylglycinol: 2d
Yield = 98%; m.p.: 114–116 °C [hexane:ethylacetate (90:10)]; [α]D = −10 (c = 0.5, CHCl3). IR (cm−1): νNH = 3302, νOH = 3477. 1H NMR (300 MHz, CDCl3): 3.35 (s, 1H); 3.72–3.75 (m, 2H); 4.07–4.59 (m, 1H); 6.25 (d, 1H); 7.04–7.37 (m, 10H). 13C NMR (75 MHz, CDCl3): (60.23, CH–NH–); (66.49, –CH2–O–); (126.76–140.56, Carom). Anal. calc. for C14H15NO3S (277.34): C, 60.63; H, 5.45; N, 5.05. Found: C, 60.53; H, 5.42; N, 5.10.
4.5 (2S)-N-(Phenylsulfonyl)phenylalaninol: 2e
Yield = 95%; m.p.: 64–66 °C [hexane:ethylacetate (90:10)]; [α]D = +18.7 (c = 0.5, CHCl3). IR (cm−1): νNH = 3193, νOH = 3416. 1H NMR (300 MHz, CDCl3): 2.64–2.83 (dd, 2H); 3.46–3.70 (m, 3H); 5.40 (d, 1H); 6.90–7.73 (m, 10H). 13C NMR (75 MHz, CDCl3): (38.10, –CH2–); (57.22, –CH–NH–); (64.36, –CH2–O–); (126.76–140.30, Carom). Anal. calc. for C15H17NO3S (291.36): C, 61.83; H, 5.88; N, 4.81. Found: C, 61.72; H, 5.80; N, 5.80.
4.6 (2S, 3S)-N-(Phenylsulfonyl)isoleucinol: 2f
Yield = 99%; m.p.: 58–60 °C [hexane:ethylacetate (90:10)]; [α]D = +25 (c = 0.6, CHCl3). IR (cm−1): νNH = 3216, νOH = 3408. 1H NMR (300 MHz, CDCl3): 0.69 (d, 3H); 0.86 (t, 3H); 1.02 (m, 2H); 1.41–1.52 (m, 2H); 2.35 (s, 1H); 3.15–3.24 (m, 1H); 3.52–3.54 (m, 2H); 5.09 (d, 1H); 7.23–7.51 (m, 3H); 7.86 (d, 2H). 13C NMR (75 MHz, CDCl3): (13.24, 22.91, 2CH3–); (25.39, 35.27, 2-CH–); (60, CH–NH–); (60.65, –CH2–O–); (127.34–142.58, Carom). Anal. calc. for C12H19NO3S (257.35): C, 56.01; H, 7.44; N, 5.44. Found: C, 56.10; H, 7.52; N, 5.42.
4.7 (2S)-N-(2-Naphthylsulfonyl)alaninol: 2g
Yield = 82%; m.p.: 82–84 °C [hexane:ethylacetate (90:10)]; [α]D = +23 (c = 1, CHCl3). IR (cm−1): νNH = 3175, νOH = 3327. 1H NMR (300 MHz, DMSO): 1.81 (d, 3H); 2.21 (s, 1H); 3.72–4.64 (m, 3H); 5.64 (s, 1H); 7.58–7.81 (m, 2H); 7.90–8.18 (m, 4H); 8.69 (s, 1H). 13C NMR (75 MHz, DMSO): (20.11, CH3–); (56.86, CH–NH–); (70.89, –CH2–O–); (119.73–13251, Carom).
4.8 (2S)-N-(2-Naphthylsulfonyl)valinol: 2h
Yield = 85%; m.p.: 96–98 °C [hexane:ethylacetate (90:10)]; [α]D = −10 (c = 1, CHCl3). IR (cm−1): νNH = 3187, νOH = 3491. 1H NMR (300 MHz, DMSO): 0.61–0.89 (2d, 6H); 2.05 (s, 1H); 3.89–4.51 (m, 3H); 5.82 (d, 1H); 7.60–7.73 (m, 2H); 7.89–8.05 (m, 4H); 8.54 (s, 1H). 13C NMR (75 MHz, DMSO): (16.27, 20.07, 2CH3–); (30.10, –CH–); (61.20, CH–NH–); (66.92, –CH2–O–); (120.12–1139.75, Carom). Anal. calc. for C15H19NO3S (293.38): C, 61.41; H, 6.53; N, 4.77. Found: C, 60.80; H, 7.10; N, 4.62.
4.9 (2S, 3S)-N-(2-Naphthylsulfonyl)isoleucinol: 2i
Yield = 86%; m.p.: 112–114 °C [hexane:ethylacetate (90:10)]; [α]D = +18 (c = 1, CHCl3). IR (cm−1): νNH = 3217, νOH = 3512. 1H NMR (300 MHz, DMSO): 0.81 (d, 3H); 0.83–0.99 (t, 3H); 1.24–1.42 (m, 2H); 1.57–2.74 (m, 1H); 2.18 (s, 1H); 3.86–4.29 (m, 3H); 5.71 (d, 1H); 7.18–7.41 (m, 2H); 7.68–8.27 (m, 4H); 8.83 (s, 1H). 13C NMR (75 MHz, DMSO): (1125, 13.08, 2CH3–); (26.24, –CH–) (36.18, –CH2–); (60.64, CH–NH–); (66.71, –CH2–O–); (122.08–139.16, Carom).
4.10 (2S)-N-(2-Naphthylsulfonyl)phenylalaninol: 2j
Yield = 75%; m.p.: 106–108 °C [hexane:ethylacetate (90:10)]; [α]D = +22 (c = 1, CHCl3). IR (cm−1): νNH = 3211, νOH = 3519. 1H NMR (300 MHz, DMSO): 2.09 (s, 1H); 2.82–2.89 (m, 1H); 3.41–3.48 (m, 1H); 3.98–4.12 (m, 2H); 4.57–4.62 (m, 1H); 5.75 (d, 1H); 7.21–7.45 (m, 5H); 7.62–7.74 (m, 2H); 8.01–8.16 (m, 4H); 8.74 (s, 1H). 13C NMR (75 MHz, DMSO): (40.21, –CH2–); (57.51, CH–N–); (67.95, –CH2–O–); (121.75–136.93, Carom).
4.11 (2S)-N-(4-Methylbenzenesulfonyl)isoleucinol: 2k
Yield = 98%; m.p.: 80–81 °C [hexane:ethylacetate (90:10)]; [α]D = −15 (c = 1, CHCl3). IR (cm−1): νNH = 3300, νOH = 3481. 1H NMR (300 MHz, CDCl3): 0.75 (d, 3H); (t, 3H); 0.91 (m, 2H); 1.35–1.49 (m, 2H); 2.2 (s, 1H); 2.42 (s, 3H); 3.09–3.13 (m, 1H); 3.55–3.56 (m, 2H); 5.1 (d, 1H); 7.28–7.79 (AA′BB′, 4H). 13C NMR (75 MHz, CDCl3): (11.72–21.91, 3CH3–); (25.60, 36.75, 2-CH–); (60.11 and 60.78, CH–NH–, –CH2–O–); (127.12–143.50, Carom). MS: C13H21NSO3; MW = 271 g/mol; m/z = 240 (C11H18NSO3+, 51%); m/z = 155 (C7H7SO2+, 67%); m/z = 91 (C7H7+, 100%).
4.12 (2S)-N-(4-Methylbenzenesulfonyl)phenylglycinol: 2l
Yield = 97%; m.p.: 93–94 °C [hexane:ethylacetate (90:10)]; [α]D = −10 (c = 1, CHCl3). IR (cm−1): νNH = 3319, νOH = 3396. 1H NMR (300 MHz, CDCl3): 2.4 (s, 3H); 2.68–2.76 (dd, 2H); 3.49–3.65 (m, 3H); 5.15 (d, 1H); 6.95–7.58 (m, 9H). 13C NMR (75 MHz, CDCl3): (21.61, CH3–); (61.24, CH–NH–); (67.08, –CH2–O–); (126.67–141.27, Carom). MS: C13H21NSO3; MW = 305 g/mol; m/z = 274 (C14H16NSO3+, 8%); m/z = 214 (C7H9NSO3+, 38%); m/z = 155 (C7H7SO2+, 50%); m/z = 91 (C7H7+, 100%).
4.13 Preparation of (4S)-3-(phenylsulfonyl)-4-benzyloxazolidin-2-one: 3a
To a solution of bis-(trichloromethyl)carbonate (0.43 g, 1.44 mmol, 0.3 eq) in CH2Cl2 (20 mL) at −78 °C was slowly added a solution of N-(phenylsulfonyl)phenylalaninol 2e (1.07 g, 3.68 mmol) in CH2Cl2 (40 mL). After 15 min of stirring, triethylamine (10 mmol, 1.4 mL, 3 eq) in CH2Cl2 (80 mL) was added dropwise maintaining the temperature below −70 °C. The resulting mixture was stirred at −78 °C for 5 min and then the ethylacetate–N2 (liquid) bath was removed. The reaction mixture was stirred at room temperature for 2 h and washed with 1 N HCl (50 mL) and brine (3 × 25). Evaporation of the solvent under reduced pressure gave a residue, which was purified by chromatography on silica gel using [cyclohexane/ethylacetate (8:2)], as mobile phase.
Yield = 96%; m.p.: 107–109 °C [hexane:ethylacetate (90:10)]; [α]D = +38.3 (c = 1, CHCl3). IR (cm−1): νCO = 1772. 1H NMR (300 MHz, CDCl3): 2.83–2.88 (m, 1H); 3.49–3.55 (m, 1H); 4.11–4.20 (m, 2H); 4.65–4.73 (m, 1H); 7.02–7.70 (m, 10H). 13C NMR (75 MHz, CDCl3): (40.13, –CH2–); (58.41, CH–N–); (67.06, –CH2–O–); (127.99–138.48, Carom); (152.38, CO). MS: C16H15NO4S; MW = 317 g/mol; m/z = 253 (C16H15NO2+, 64%); m/z = 141 (C6H5O2S+, 81%); m/z = 91 (C7H7+, 100%); m/z = 77 (C6H5+, 43%). Anal. calc. for C16H15NO4S (317.36): C, 60.55; H, 4.76; N, 4.41. Found: C, 60.10; H, 4.32; N, 4.20.
4.14 (4S)-3-(Phenylsulfonyl)-4-i-propyloxazolidin-2-one: 3b
Compound 3b (90:10); [α]D = +58.8 (c = 0.5, CHCl3). IR (cm−1): νCO = 1761. 1H NMR (300 MHz, CDCl3): 0.70–0.94 (2d, 6H); 2.41–2.52 (m, 1H); 4.14–4.48 (m, 3H); 7.54–8.11 (m, 5H). 13C NMR (75 MHz, CDCl3): (14.30, 18.16, 30.24, 3CH3–); (62.08, CH–NH–); (63.90, –CH2–O–); (128.74–138.46, Carom); (152.79, CO). MS: C12H15NO4S; MW = 269 g/mol; m/z = 226 (C9H8NO4S+, 38%); m/z = 205 (C12H15NO2+, 12%); m/z = 176 (C9H8NO2+, 31%); m/z = 141 (C7H7SO2+, 100%); m/z = 77 (C6H5+, 67%). Anal. calc. for C12H15NO4S (269.32): C, 53.52; H, 5.61; N, 5.20. Found: C, 53.40; H, 5.52; N, 5.13.
4.15 (4S)-3-(Phenylsulfonyl)-4-methyloxazolidin-2-one: 3c
Yield = 95%; m.p.: 60–62 °C [hexane:ethylacetate (90:10)]; [α]D = +45 (c = 1, CHCl3). IR (cm−1): νCO = 1761. 1H NMR (300 MHz, CDCl3): 1.48 (d, 3H); 3.87–3.91 (m, 1H); 4.37–4.56 (m, 2H); 7.50–8.03 (m, 5H). 13C NMR (75 MHz, CDCl3): (20.66, CH3–); (53.71, CH–NH–); (69.70, –CH2–O–); (128.31–138.05, Carom); (152.17, CO). MS: C10H11NO4S; MW = 241 g/mol; m/z = 241 M+, 7%); m/z = 226 (C9H8NO4S+, 30%); m/z = 177 (C10H11NO2+, 55%); m/z = 141 (C6H5SO2+, 100%); m/z = 77 (C6H5+, 57%). Anal. calc. for C10H11NO4S (241.26): C, 49.78; H, 4.60; N, 5.81. Found: C, 49.72; H, 4.53; N, 5.60.
Enantiomeric purity of 3c was determined by HPLC analyses on Chirobiotic V column (250 × 46 mm) with a flow rate of 0.6 mL/min. Mobile phase methanol/hexane [70:30]; retention times: (4S)-3-(phenylsulfonyl)-4-methyloxazolidin-2-one 10.4 min; (4R)-3-(phenylsulfonyl)-4-methyloxazolidin-2-one 15.2 min.
4.16 (4S)-3-(Phenylsulfonyl)-4-i-butyloxazolidin-2-one: 3d
Yield = 96%; m.p.: 139–141 °C [hexane:ethylacetate (90:10)]; [α]D = +39.6 (c = 0.5, CHCl3). IR (cm−1): νCO = 1770. 1H NMR (300 MHz, CDCl3): 0.90–1.05 (m, 6H); 1.56–1.67 (m, 2H); 1.94–2.04 (m, 1H); 4.05–4.09 (m, 1H); 4.36–452 (m, 2H); 7.54–8.08 (m, 5H). 13C NMR (75 MHz, CDCl3): (21.44, 23.61, 24.67, 3CH3–); (42.87, –CH2–); (56.27, CH–NH–); (68.15, –CH2–O–); (128.41–138.21, Carom); (152, CO). MS: C13H17NO4S; MW = 283 g/mol; m/z = 219 (C13H17NO2+, 13%); m/z = 162 (C9H8NO2+, 24%); m/z = 141 (C6H5SO2+, 100%); m/z = 77 (C6H5+, 67%). Anal. calc. for C13H17NO4S (283.34): C, 55.11; H, 6.05; N, 494. Found: C, 55.01; H, 6.17; N, 4.83.
4.17 (4R)-3-(Phenylsulfonyl)-4-phenyloxazolidin-2-one: 3e
Yield = 97%; m.p.: 118–120 °C [hexane:ethylacetate (90:10)]; [α]D = −13.6 (c = 1, CHCl3). IR (cm−1): νCO = 1775. 1H NMR (300 MHz, CDCl3): 4.27–4.31 (m, 1H); 4.71–4.77 (t, 1H); 5.42–5.46 (m, 1H); 7.19–7.55 (m, 10H). 13C NMR (75 MHz, CDCl3): (60.41, CH–NH–); (70.51, –CH2–O–); (127.12–137.68, Carom); (152.02, CO). MS: C15H13NO4S; MW = 303 g/mol; m/z = 239 (C15H13O2N+, 65%); m/z = 141 (C6H5O2S+, 100%); m/z = 77 (C6H5+, 58%). Anal. calc. for C15H13NO4S (303.33): C, 59.40; H, 4.32; N, 4.62. Found: C, 59.20; H, 4.21; N, 4.65.
4.18 (4S)-3-(Phenylsulfonyl)-4-[(1′S)-1′-methylpropyl]oxazolidin-2-one: 3f
Yield = 95%; m.p.: 144–146 °C [hexane:ethylacetate (90:10)]; [α]D = 60 (c = 1, CHCl3). IR (cm−1): νCO = 1772. 1H NMR (300 MHz, CDCl3): 0.72 (d, 3H); 0.96–1.00 (t, 3H); 1.09–1.34 (m, 2H); 2.22 (m, 1H); 4.12–4.16 (m, 1H); 4.25–4.31 (t, 1H); 4.53–4.56 (m, 1H); 7.55–8.11 (m, 5H). 13C NMR (75 MHz, CDCl3): (11.68, 12.19, 2CH3–); (25.65, –CH–); (37.05, –CH2–); (61.05, CH–NH–); (63.94, –CH2–O–); (128.76–138.40, Carom); (152.87, CO). MS: C13H17NO4S; MW = 283 g/mol; m/z = 219 (C13H17NO2+, 17%); m/z = 226 (C9H8NO4S+, 15%); m/z = 141 (C6H5SO2+, 100%); m/z = 77 (C6H5+, 63%). Anal. calc. for C13H17NO4S (283.34): C, 55.11; H, 6.05; N, 4.94. Found: C, 55.10; H, 5.76; N, 4.80.
4.19 (4S)-3-(2-Naphthylsulfonyl)-4-methyloxazolidin-2-one: 3g
Yield = 88%; m.p.: 129–131 °C [hexane:ethylacetate (90:10)]; [α]D = +40.1 (c = 0.5, CHCl3). IR (cm−1): νCO = 1762. 1H NMR (300 MHz, CDCl3): 1.59 (d, 3H); 3.93–4.66 (m, 3H); 7.62–7.73 (m, 2H); 7.92–8.05 (m, 4H); 8.68 (s, 1H). 13C NMR (75 MHz, CDCl3): (21.19, CH3–); (54.04, CH–NH–); (69.96, –CH2–O–); (122.94–135.94, Carom); (152.47, CO). MS: C14H13NO4S; MW = 291 g/mol; m/z = 291 (M+, 13%); m/z = 227 (C14H13NO2+, 35%); m/z = 168 (C12H10N+, 26%); m/z = 127 (C10H7+, 100%). Anal. calc. for C14H13NO4S (291.32): C, 57.72; H, 4.50; N, 4.81. Found: C, 57.62; H, 4.41; N, 4.62.
4.20 (4S)-3-(2-Naphthylsulfonyl)-4-i-propyloxazolidin-2-one: 3h
Yield = 85%; m.p.: 141–143 °C [hexane:ethylacetate (90:10)]; [α]D = +16.7 (c = 0.5, CHCl3). IR (cm−1): νCO = 1766. 1H NMR (300 MHz, CDCl3): 0.74–0.97 (2d, 6H); 4.14–4.53 (m, 3H); 7.61–7.72 (m, 2H); 7.91–8.02 (m, 4H); 8.68 (s, 1H). 13C NMR (75 MHz, CDCl3): (14.39, 18.17, 2CH3–); (30.13, –CH–); (62.14, CH–NH–); (63.94, –CH2–O–); (122.57–135.91, Carom); (152.87, CO). MS: C17H19NO4S; MW = 333 g/mol; m/z = 319 (M+, 11%); m/z = 212 (C13H10NO2+, 31%); m/z = 191 (C10H7NO2S+, 52%); m/z = 127 (C10H7+, 100%). Anal. calc. for C16H17NO4S (319.38): C, 60.17; H, 5.37; N, 4.39. Found: C, 59.95; H, 5.11; N, 4.44.
Enantiomeric purity of 3c was determined by HPLC analyses on Chirobiotic V column (250 × 46 mm) with a flow rate of 0.6 mL/min. Mobile phase methanol/hexane [70:30]; retention times: (4S)-3-(2-naphthylsulfonyl)-4-i-propyloxazolidin-2-one 11.2 min; (4R)-3-(2-naphthylsulfonyl)-4-i-propyloxazolidin-2-one 13.3 min.
4.21 (4S)-3-(2-Naphthylsulfonyl)-4-[(1′S)-1′-methylpropyl]oxazolidin-2-one: 3i
Yield = 87%; m.p.: 130–132 °C [hexane:ethylacetate (90:10)]; [α]D = +60 (c = 1, CHCl3). IR (cm−1): νCO = 1776. 1H NMR (300 MHz, CDCl3): 0.74 (d, 3H); 0.98–1.02 (t, 3H); 1.16–1.37 (m, 2H); 2.04–2.17 (m, 1H); 4.13–4.61 (m, 3H); 7.12–7.15 (m, 2H); 7.62–8.05 (m, 4H); 8.69 (s, 1H). 13C NMR (75 MHz, CDCl3): (11.75, 12.11, 2CH3–); (25.68, –CH–); (37.20, –CH2–); (61.11, CH–NH–); (63.94, –CH2–O–); (122.93–135.91, Carom); (152.93, CO). MS: C17H19NO4S; MW = 333 g/mol; m/z = 333 (M+, 7%); m/z = 212 (C13H10NO2+, 23%); m/z = 191 (C10H7NO2S+, 64%); m/z = 127 (C10H7+, 100%). Anal. calc. for C17H19NO4S (333.40): C, 61.24; H, 5.74; N, 4.20. Found: C, 61.20; H, 5.72; N, 4.10.
4.22 (4S)-3-(2-Naphthylsulfonyl)-4-benzyloxazolidin-2-one: 3j
Yield = 79%; m.p.: 107–109 °C [hexane:ethylacetate (90:10)]; [α]D = +71.4 (c = 0.5, CHCl3). IR (cm−1): νCO = 1773. 1H NMR (300 MHz, CDCl3): 2.84–2.93 (m, 1H); 3.57–3.63 (m, 1H); 4.10–4.27 (m, 2H); 4.71–4.80 (m, 1H); 7.15–7.39 (m, 5H); 7.64–7.75 (m, 2H); 7.95–8.11 (m, 4H); 8.74 (s, 1H). 13C NMR (75 MHz, CDCl3): (40.26, –CH2–); (58.49, CH–N–); (67.05, –CH2–O–); (122.95–135.99, Carom); (152.42, CO). MS: C17H19NO4S; MW = 367 g/mol; m/z = 212 (C13H10NO2+, 15%); m/z = 191 (C10H7NO2S+, 72%); m/z = 127 (C10H7+, 100%); m/z = 91 (C7H7+, 100%). Anal. calc. for C20H17NO4S (367.42): C, 65.38; H, 4.66; N, 3.81. Found: C, 65.32; H, 4.62; N, 3.82.
4.23 (4S)-3-(4-Methylbenzenesulfonyl)-4-i-propyloxazolidin-2-one: 3k
Yield = 95%; m.p.: 115–117 °C [hexane:ethylacetate (90:10)]; [α]D = +58.2 (c = 0.5, CHCl3). IR (cm−1): νCO = 1770. 1H NMR (300 MHz, CDCl3): 0.72–0.92 (2d, 6H); 2.43 (s, 3H); 4.12–4.45 (m, 3H); 7.32–7.96 (AA′BB′, 4H). 13C NMR (75 MHz, CDCl3): (14.3–22, 3CH3–); (30.26, –CH–); (62.05, CH–NH–); (63.91, –CH2–O–); (128.7–147.9, Carom); (152.8, CO). MS: C13H17NSO4; MW = 283 g/mol; m/z = 240 (C10H10NSO4+, 13%); m/z = 219 (C13H17NO2+, 13%); m/z = 176 (C10H10NO2+, 33%); m/z = 155 (C7H7SO2+, 100%); m/z = 91 (C7H7+, 88%). Anal. calc. for C13H17NO4S (283.34): C, 55.11; H, 6.05; N, 4.94. Found: C, 55.40; H, 5.80; N, 4.70.
4.24 (4S)-3-(4-Methylbenzenesulfonyl)-4-i-butyloxazolidin-2-one: 3l
Yield = 96%; m.p.: 151–153 °C [hexane:ethylacetate (90:10)]; [α]D = +38.4 (c = 0.5 CHCl3). IR (cm−1): νCO = 1768. 1H NMR (300 MHz, CDCl3): 0.95–0.98 (m, 6H); 1.58–1.64 (m, 2H); 1.97 (m, 1H); 2.43 (s, 3H); 4.03–4.45 (m, 3H); 7.33–7.95 (AA′BB′, 4H). 13C NMR (75 MHz, CDCl3): (21.7–25.06, 3CH3–); (43.19, –CH2–); (56.59, CH–NH–); (68.48, –CH2–O–); (128.81–145.94, Carom); (152.66, CO). MS: C14H19NSO4; MW = 297 g/mol; m/z = 233 (C14H19NO2+, 7%); m/z = 176 (C10H10NO2+, 15%); m/z = 155 (C7H7SO2+, 100%); m/z = 91 (C7H7+, 71%). Anal. calc. for C14H19NO4S (297.37): C, 56.55; H, 6.44; N, 4.71. Found: C, 55.30; H, 6.32; N, 4.60.
4.25 (4S)-3-(4-Methylbenzenesulfonyl)-4-[(1′S)-1′-methylpropyl]oxazolidin-2-one: 3m
Yield = 95%; m.p.: 170–172 °C [hexane:ethylacetate (90:10)]; [α]D = +41 (c = 1, CHCl3). IR (cm−1): νCO = 1774. 1H NMR (300 MHz, CDCl3): 0.72 (d, 3H); 0.96 (t, 2H); 2.43 (s, 3H); 4.09–4.54 (m, 3H); 7.32–7.96 (AA′BB′, 4H). 13C NMR (75 MHz, CDCl3): (11.54, 11.90, 2CH3–); (25.51, –CH–) (36.89, –CH2–); (61.31, CH–NH–); (63.82, –CH2–O–); (127.32–138.41, Carom); (152.75, CO). MS: C14H19NSO4; MW = 297 g/mol; m/z = 233 (C14H19NO2+, 10%); m/z = 240 (C10H10NSO4+, 18%); m/z = 155 (C7H7SO2+, 100%); m/z = 91 (C7H7+, 58%). Anal. calc. for C14H19NO4S (297.37): C, 56.55; H, 6.44; N, 4.71. Found: C, 56.50; H, 6.42; N, 4.62.
4.26 (4S)-3-(4-Methylbenzenesulfonyl)-4-methyloxazolidin-2-one: 3n
Yield = 97%; m.p.: 117–119 °C [hexane:ethylacetate (90:10)]; [α]D = +52.2 (c = 0.5, CHCl3). IR (cm−1): νCO = 1779. 1H NMR (300 MHz, CDCl3): 1.52 (d, 3H); 2.4 (s, 3H); 3.89–4.57 (m, 3H); 5.15 (d, 1H); 7.33–7.95 (AA′BB′, 4H). 13C NMR (75 MHz, CDCl3): (21–22, 2CH3–); (53.96, CH–NH–); (69.92, –CH2–O–); (128.71–150.95, Carom); (152.50, CO). MS: C11H13NSO4; MW = 255 g/mol; m/z = 255 (M+, 4%); m/z = 254 (C11H12NSO4+, 32%); m/z = 191 (C11H13NO2+, 50%); m/z = 91 (C7H7+, 73%); m/z = 64 (SO2+, 74%). Anal. calc. for C11H13NO4S (255.29): C, 51.75; H, 5.13; N, 5.49. Found: C, 51.70; H, 5.20; N, 5.40.
4.27 (4R)-3-(4-Methylbenzenesulfonyl)-4-phenyloxazolidin-2-one: 3o
Yield = 95%; m.p.:149–151 °C [hexane:ethylacetate (90:10)]; [α]D = −39.5 (c = 0.5, CHCl3). IR (cm−1): νCO = 1774. 1H NMR (300 MHz, CDCl3): 2.37 (d, 3H); 4.25–4.29 (m, 1H); 5.41–5.43 (m, 1H); 7.09–7.31 (m, 9H). 13C NMR (75 MHz, CDCl3): (22.01, 1CH3–); (60.75, CH–NH–); (70.87, –CH2–O–); (127.41–145.59, Carom); (152.45, CO). MS: C16H15NSO4; MW = 317 g/mol; m/z = 253 (C16H15NO2+, 71%); m/z = 91 (C7H7+, 100%); m/z = 77 (C6H5+, 55%). Anal. calc. for C16H15NO4S (317.36): C, 60.55; H, 4.76; N, 4.41. Found: C, 60.52; H, 4.72; N, 4.25.
4.28 (4S)-3-(4-Methylbenzenesulfonyl)-4-benzyloxazolidin-2-one: 3p
Yield = 96%; m.p.: 136–138 °C [hexane:ethylacetate (90:10)]; [α]D = +36.2 (c = 0.5, CHCl3). IR (cm−1): νCO = 1774. 1H NMR (300 MHz, CDCl3): 2.37 (d, 3H); 4.25–4.29 (m, 1H); 5.41–5.43 (m, 1H); 7.09–7.31 (m, 9H). 13C NMR (75 MHz, CDCl3): (22, 1CH3–); (41.25, –CH2–); (60.7, CH–NH–); (70.8, –CH2–O–); (127.40–145.59, Carom); (152.45, CO). MS: C16H15NO4S; MW = 317 g/mol; m/z = 253 (C16H15NO2+, 71%); m/z = 155 (C7H7SO2+, 10%); m/z = 91 (C7H7+, 100%); m/z = 77 (C6H5+, 55%). Anal. calc. for C17H17NO4S (331.39): C, 61.62; H, 5.17; N, 4.23. Found: C, 61.50; H, 5.20; N, 4.20.
Acknowledgments
We are grateful to the DGRSRT (the Direction générale de la Recherche scientifique et de la Rénovation technologique) for grants to ‘Laboratoire de synthèse organique asymétrique et catalyse homogène’.


