1 Experimental
Crown ethers are effective extractants due to their ability to form stable complexes with metal ions. This property of crown ethers acquired especial importance for the elaboration of new processes to extract radioactive elements from solutions with complicated chemical composition obtained after the dissolution of irradiated nuclear fuel and highly radioactive wastes.
The most difficult problem is the separation of transplutonium elements (TPE) and REE in +3 oxidation state. Solvent extraction and sorption methods of TPE and REE separation, including application of crown ethers, were reviewed [1]. Solvent extraction of lanthanide elements by various crown ethers containing 15–21 atoms in the cycle from nitric acid solutions into benzene was studied [2]. The best results were obtained for solvent extraction of Nd (D = 0.615) by 1 M 18-crown-6 with cyclohexano fragments (DCH18C6) and salting out by 8 M NH4NO3. These results did not allow one to conclude about the selectivity of extraction and the composition of the extracted complexes. Solvent extraction of REE by crown ethers in the presence of picrate-ions is also described in a paper [3]. It was found that extraction constants decreased with the increase of the atomic number of REE, excluding La. The extraction system to separate light actinides (Ac) from heavier ones based on the use of 18-crown-6 as the neutral macrocycle ligand and trichloroacetate as the counter ion is proposed in Ref. [4].
The use of chelate extractants such as β-diketons, pyrozolons, acidic organophosphorous extractants or others and crown ethers as neutral adducts was applied for synergistic extraction in order to improve extraction and selective properties of REE and TPE. Such synergistic extraction of Ln by a mixture of 18-crown-6 or dicyclohexano-18-crown-6 with TTA was studied in a paper [5]. Considerable enhancement of extraction ability and selectivity of lighter REE was found, which offers the possibility to develop effective methods for separation of lanthanides(III). Synergistic extraction of REE and TEE with crown ethers and TTA was described in a paper [6]. It was noticed that the correlation between the sizes of the cations and the cavern cavity of crown ethers was not an important factor for complexation with TTA, which was explained by means of interaction of metal ions only with a few potential oxygen donorsi steric effects are important for this number. The increase in intergroup extraction and intergroup separation of Eu, Am, Cm and Cf by the mixture of 1-phenyl-3-methyl-4-trifluoroacetylpyrazolone-5 and crown ethers in CHCl3 was reached using chloroacetate buffer as the aqueous phase [7–9].
Two series of 18-crown-6 with phenyl (I) and cyclohexano (II) fragments in the polyether ring and the alkyl side substituent in the periphery of these fragments are considered in the current paper: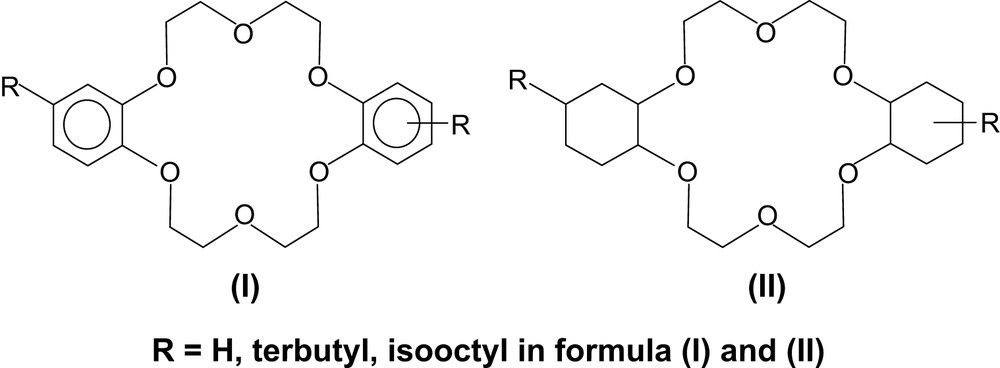
The studied crown ethers were synthesized by original techniques [8] and were of not less than 98% purity according to the data of chromatography analysis.
Distribution coefficients DM were used as the measure of extraction ability of alkyl derivatives of 18-crown-6 at the simultaneous extraction of 15 REE (excluding Pm), including La and Y. Separation factors are expressed as βMe1/Me2 = D1/D2, where D1 and D2 are distribution coefficients of M1 and M2, respectively, which were used to estimate the seperation effectivity. Solutions of the sum with all the REE, used for investigation of the extraction, were obtained by diluting the initial individual solution, containing 1 g/L of each metal, in a 1 M HNO3. The initial individual solution was diluted by especially pure water with addition of respective acids down to 100 mg/L concentration of each metal. Nitric, perchloric and trichloroacetic (TCAA) acids were used for the experiments. The stripping was carried out by especially pure water after 3 min of shaking and phase separation. Concentrations of metals in the stripping phase were determined by ICP-AES method, using IRIS Advantage device of Thermo Jarrell Ash Co. An aliquot of 241Am(III) was added to the respective aqueous phase, containing 100 mg/mL of each REE and acids, and shaken for 3 min with the equal volume of organic phase in experiments to study the behaviour of Am(III). The phases were separated after centrifugation and aliquot parts were taken to measure γ-activity. DAm values were designed by these data. All experiments were carried out at room temperature.
It was found that no extraction of REE and Am(III) in the presence of pure nitric and perchloric acids took place. These results can be related to all the studied crown ethers: dicyclohexano-18-crown-6 (DCH18C6) and diterbutyl (DTBDCH18C6) and diisooctyl (DIODCH18C6) derivatives of the latter and to diterbutyl (DTBDB18C6) and diisooctyl (DIODB18C6) derivatives.
Interesting extraction results were obtained in the presence of TCAA. It was found that only DCH18C6 and its derivatives have extraction ability, whereas the extraction by DB18C6 and the derivatives of the latter is insufficient (rare-earth metals' concentration in the stripping phase was lower than the detection limit).
The best results for extraction of sum of REE by DTBDCH18C6 in the presence of TCAA are shown in Figs. 1 and 2. As is seen from the figures, metals of the Ce subgroup are extracted quite well (Fig. 1), contrary to metals of the Y subgroup (Fig. 2). The extraction of Am(III) was carried out under similar conditions by 0.05 M solutions of DCH18C6, DIODCH18C6 and DTBDCH18C6B in CHCl3 at various concentrations of TCAA (Fig. 3). It is seen from the figure that at a TCAA concentration below around 1 M the extraction by DTBDCH18C6 takes place between the extraction by DCH18C6 and DIODCH18C. The ligand DTBDCH18C6 gives the best extraction properties when the TCAA concentration is higher than 1 M. It is necessary to notice here that the values of DLn become lower with the increase of the atomic order number in the row of REE, either for the Ce subgroup or for the Y one. The values obtained for Y subgroup REE are values of two orders lower than for the Ce subgroup. Isotherms were not shown for all metals to avoid complicated figures. Separation factors for well-extracted metals of the Ce subgroup relatively to each other and relatively slightly extracted Yb are shown in Table 1. These separation factors are seen to be high enough.
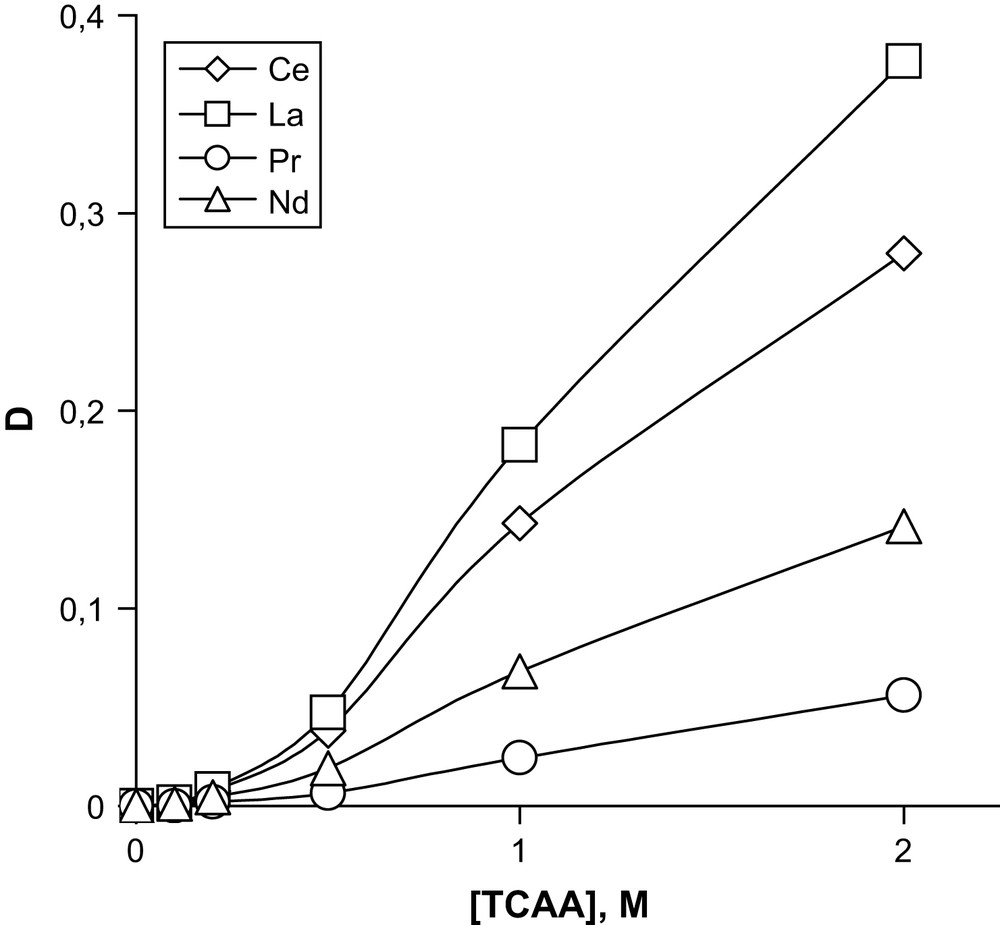
Dependence of distribution coefficients for the extraction of REE of Ce subgroup by 0.05 M DTBDCH18C6 in CHCl3 on the concentration of TCAA. Aqueous phase contains a sum of REE in 1.0 M HNO3.
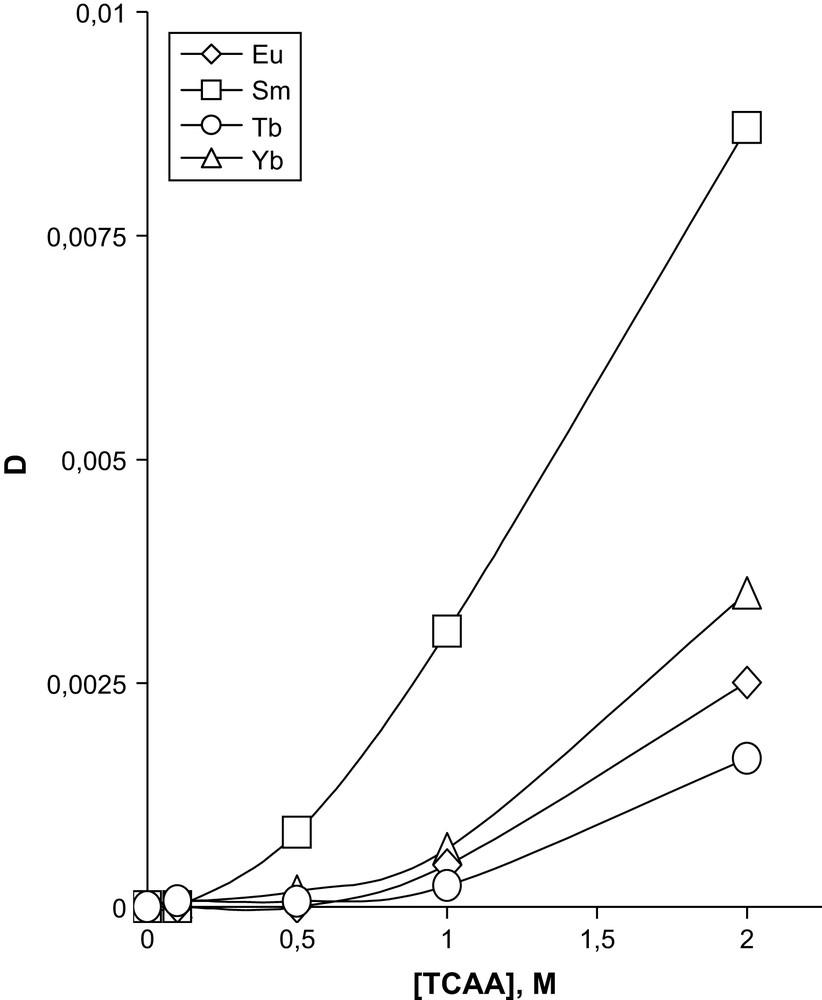
Dependence of distribution coefficients for the extraction of REE of Y subgroup by 0.05 M DTBDCH18C6 in CHCl3 on the concentration of TCAA. Aqueous phase contains a sum of REE in 1.0 M HNO3.
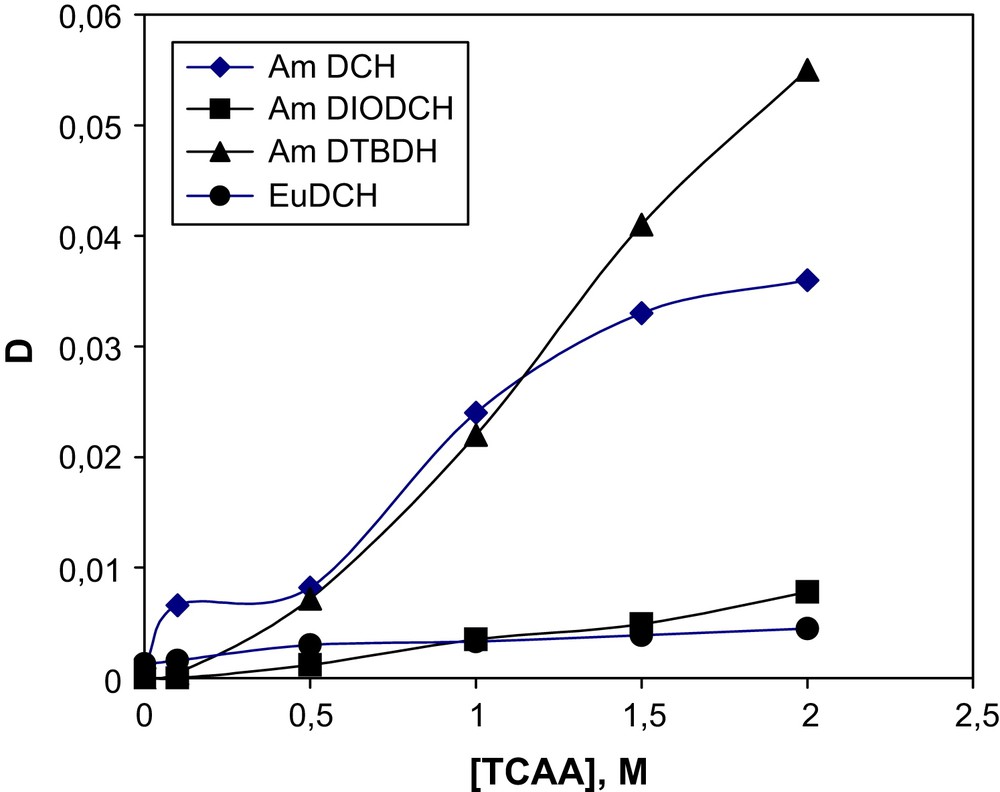
Dependence of Am(III) and Eu(III) distribution coefficients for the extraction by 0.05 M of DCH18C6, DIODCH18C6 and DTBDCH18C6 in CHCl3 on the concentration of TCAA. The aqueous phase contains a sum of REE with either 241Am(III) or 152–154Eu(III) in 1.0 M HNO3.
Separation factors of REE during extraction from HNO3 in the presence of 1 M TCAA by 0.05 M by crown ethers in CHCl3
| Crown-ether | [HNO3] (M) | Distribution factors | |||||||||
| La/Ce | La/Pr | La/Nd | La/Yb | Ce/Pr | Ce/Nd | Ce/Yb | Pr/Nd | Pr/Yb | Nd/Yb | ||
| DCH | 0.1 | 0.88 | 1.38 | 2.76 | 300 | 1.57 | 3.14 | 341 | 2.00 | 217 | 109 |
| 0.5 | 0.92 | 1.39 | 2.37 | 153 | 1.52 | 2.59 | 167 | 1.71 | 110 | 64.5 | |
| DIODCH | 0.1 | 1.29 | 2.27 | 4.26 | 8.39 | 1.77 | 3.30 | 6.50 | 1.87 | 3.68 | 1.98 |
| 0.5 | 1.19 | 2.15 | 3.88 | 18.7 | 1.80 | 3.25 | 15.6 | 1.81 | 8.69 | 4.81 | |
| DTBDCH | 0.1 | 1.28 | 2.69 | 7.49 | 777 | 2.10 | 5.86 | 608 | 2.79 | 289 | 104 |
The mutual influence of different kinds of acids on the extraction of Am(III) and REE was also investigated. The influence of HNO3 concentration on the extraction of REE in the presence of 1 M TCAA is shown in Fig. 4. The extraction of Am(III) by DCH18C6, DIODCH18C6 and DTBDCH18C6 is represented in Fig. 5. It is seen that extraction of Am(III) and lanthanides falls down with an increase in HNO3 concentration higher than 0.1 M, whereas the separation factors changed slightly.

Dependence of distribution coefficients for the extraction of REE by 0.05 M DCH18C6 and DIODCH18C6 in CHCl3 in the presence of 1 M TCAA on HNO3 concentration.
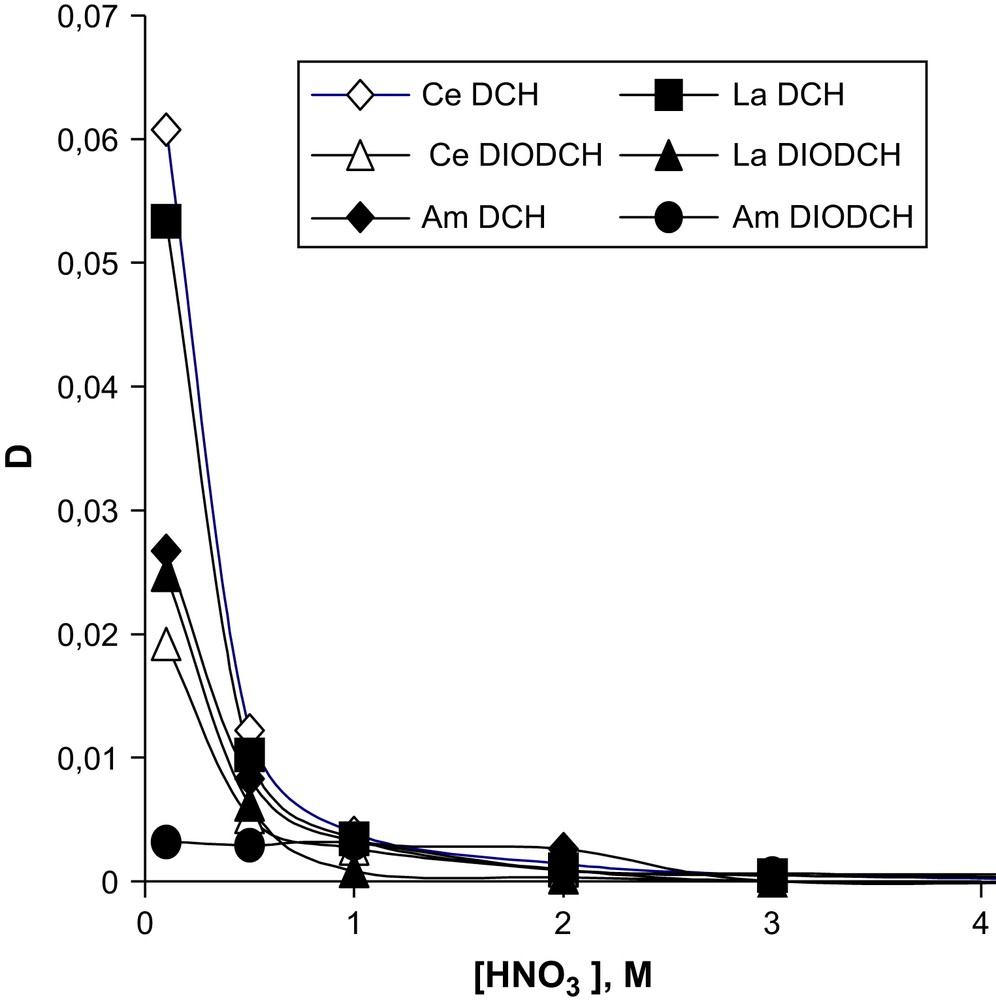
Dependence of Am(III) distribution coefficients for the extraction by 0.05 M DCH18C6, DIODCH18C6 and DTBDCH18C6 in CHCl3 in the presence of 1 M of TCAA on HNO3 concentration. Aqueous phase contains a sum of REE with Am(III).
The study of TCAA distribution was carried out in the process of REE extraction. The concentration of REE was determined by means of titration of the stripping phase by 0.1 and 0.01 M NaHCO3. It was found that the TCAA distribution between aqueous and organic phases did not depend on the type, nature and concentration of crown ethers and on REE concentration, and is coherent with published data [10]. The concentration of TCAA in organic phase was considerably less than the sum of REE concentration.
The classical method of shift of extraction equilibrium slope analysis was applied to determine the stoichiometry of complexes.
The extraction process can be expressed by the following equilibrium equation:
Then, the extraction constant can be written as:
Hence, the following linear dependence can be obtained as a result of transformation:
The composition stoichiometry of the extracted complexes was determined by the tangent of the angle, the slope of the curve log(D/[A−]maq) versus log[L]initial. In our case mco ≪ [L]initial. The dependence of log(D/[A−]maq) on log [L]o for the extraction of the sum of rare earths by DTBDCH18C6 is shown in Fig. 6. It is seen from the figure that n = 1.
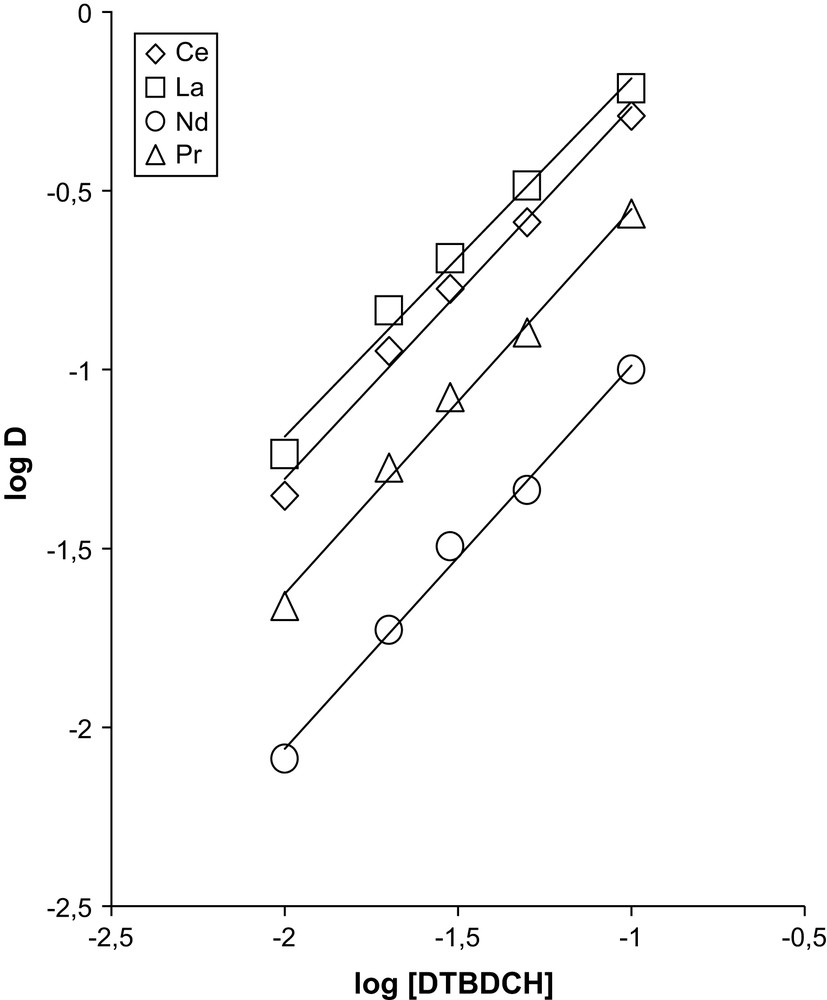
Dependence of distribution coefficients for the extraction of REE of the Ce subgroup in the presence of 1 M TCAA on DTBDCH18C6 concentration. The aqueous phase contains a sum of REE of the Ce subgroup.
The extraction of 241Am and 152–154Eu by DCH18C6 and DTBDCH18C6 in CHCl3 was studied in the presence of 1 M TCAA varying the concentration of crown ethers (Fig. 7). It is seen that Am(III) forms complexes of 1:1 composition with these crown ethers, as in the case of rare-earth metals. If the concentration of crown ethers is above 0.1 M, the distribution coefficient of Am(III) stays at a constant value without any effect of ligand concentration, which allows one to separate Am and Eu on extracting by 0.1 M DCH18C6 and 0.1 M DTBDCH18C6 with the separation factors 40 and 59, respectively.
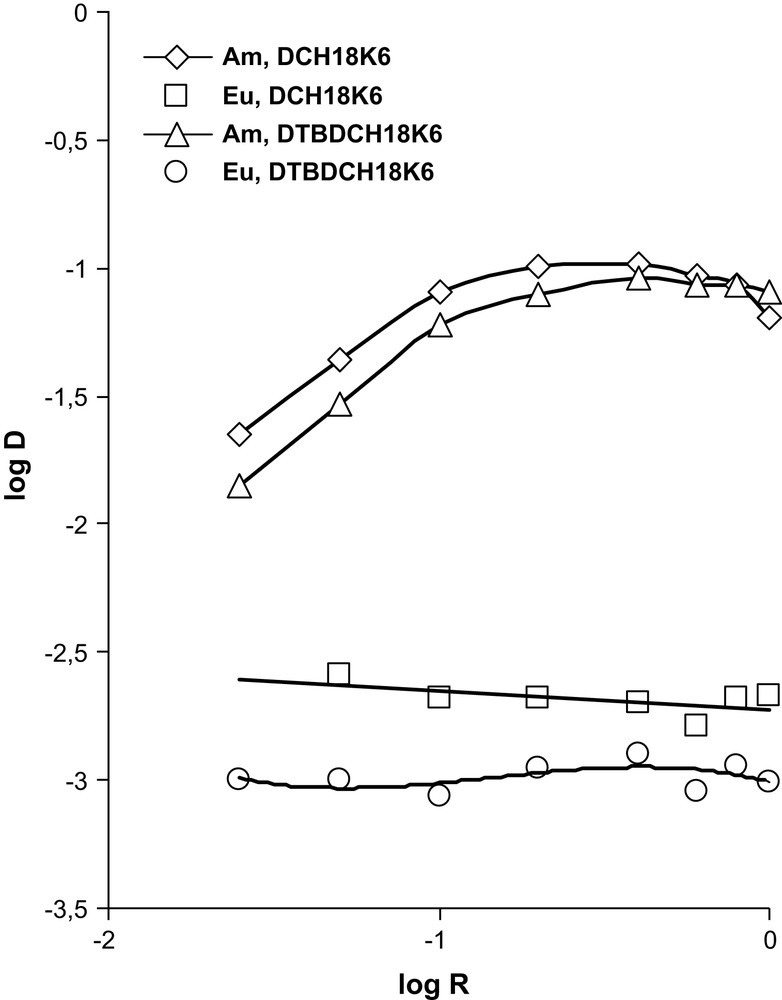
Dependence of distribution coefficients for the extraction of Am and Eu on the crown ethers' concentration in CHCl3. Aqueous phase : sum of REE in 1.0 M HNO3 in the presence of 1.0 M TCAA.
The carried-out investigations showed that the use of alkyl-substituted derivatives of DCH18C6 allows one to extract rare-earth metals of the Ce subgroup in order to separate them from each other and from rare-earth metals of the Y subgroup, which develops considerably the possibilities of extraction and separation of rare-earth metals.
Acknowledgements
The work was supported by the U.S. Department of Energy, Office of Basic Energy Sciences, Project RUC2-20010-MO04.


