1 Introduction
Effluents from the textile industry are one of the major sources of water pollution. Hence, treatment of dye wastewater has become an important task for environmental managers. While there are many methods available for treating dye wastewater, adsorption has been found to be an efficient one [1,2].
Activated carbon exhibits high adsorption capacity for organic dyes. However, its relatively high cost makes it less attractive economically. Hence, there is a need for a cheaper, but comparably effective adsorbent material. Bentonite is a promising alternative adsorbent. Numerous studies have been carried out to investigate the effectiveness of bentonite in removing pollutants [3–6]. In the current study, we report on the ability of natural bentonite (Ca-bentonite) and Na-bentonite to remove the dye Basic Violet (BV14) by adsorption from aqueous solution. Kinetics and isotherm studies were conducted to evaluate the adsorption capacity of bentonite. The aim of this work was to investigate the possibility of using bentonite, which is available in large quantities in Guangxi Autonomous Region of China, as a low-cost adsorbent for the removal of BV14.
2 Materials and methods
2.1 Materials
The natural bentonite (Ca-bentonite) of industrial grade was purchased from Ningmin County, Guangxi, China, and its chemical composition, as reported by the supplier, is shown in Table 1. Chemical-grade BV14 was purchased from Tianjin Chemicals Ltd. Co. and used without further purification. The structure of BV14 is shown in Fig. 1.
The chemical composition of natural bentonite
| Constituents | SiO2 | Al2O3 | TiO2 | Fe2O3 | CaO | MgO | K2O | Na2O |
| Percentage (%) | 64.53 | 17.86 | 0.38 | 3.23 | 1.69 | 3.05 | 1.17 | 1.27 |
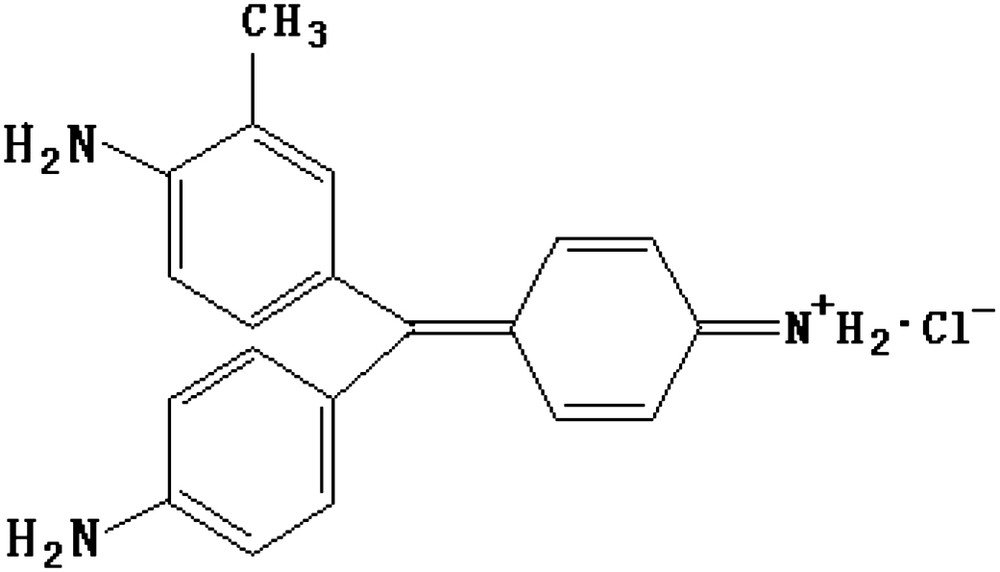
The structure of BV14.
2.2 Adsorbent
Ca-bentonite was purified by a previously reported sedimentation technique [7]. Na-bentonite was obtained by treating Ca-bentonite with three successive NaCl solutions. Purified Ca-bentonite (10 g) was dispersed in 400 mL of distilled water to make a clay suspension, to which was added 100 mL of 1 M NaCl solution at 80 °C for 2 h, followed by filtration and washing in distilled water. This cation exchanging process was repeated three times, and the final sample was dried at 90 °C for 2 h.
The cation exchange capacities (CECs) of Ca-bentonite and Na-bentonite, characterized by the methylene blue method [8], were determined to be 0.65 and 1.15 mmol g−1, respectively.
2.3 Sample characterization
X-ray powder diffraction patterns were recorded on a Rigaku D/MAX 2500V diffractometer with a copper tube as radiation source (λ = 1.54178 Å) and operating at 40 kV and 120 mA. Its profiles were recorded at 2° (2θ) per minute.
2.4 Adsorption experiments
Adsorption experiments were carried out at 25 °C. BV14 solution (100 mL) of known initial concentration was shaken with 0.5 g adsorbent at pH 6 in a temperature-controlled shaker. At various time intervals throughout the adsorption, samples were taken, and the adsorbent was separated by centrifugation. The supernate was analyzed for residual concentration of BV14 by spectroscopic analysis at the wavelengths of 555 nm, using a UV-2501PC spectrophotometer (Shimadzu).
The amount of adsorbed dye per unit mass of adsorbent at time t, qt, was then determined by the mass-balance equation:
| (1) |
3 Results and discussion
3.1 Effect of experimental conditions on adsorption processes
3.1.1 Influence of the initial concentration of BV14
The effect of the initial concentration of BV14 in the solutions on the amount of adsorbed dye was studied and the results are shown in Figs. 2 and 3.
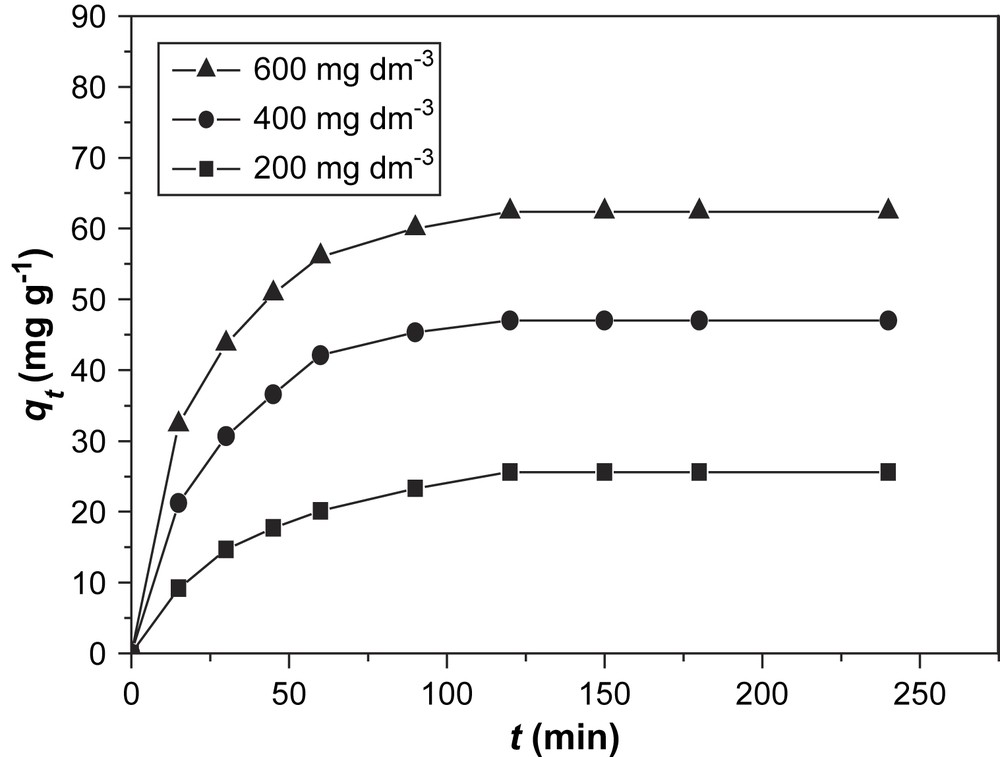
Effect of initial concentration on adsorption of BV14 on Ca-bentonite.
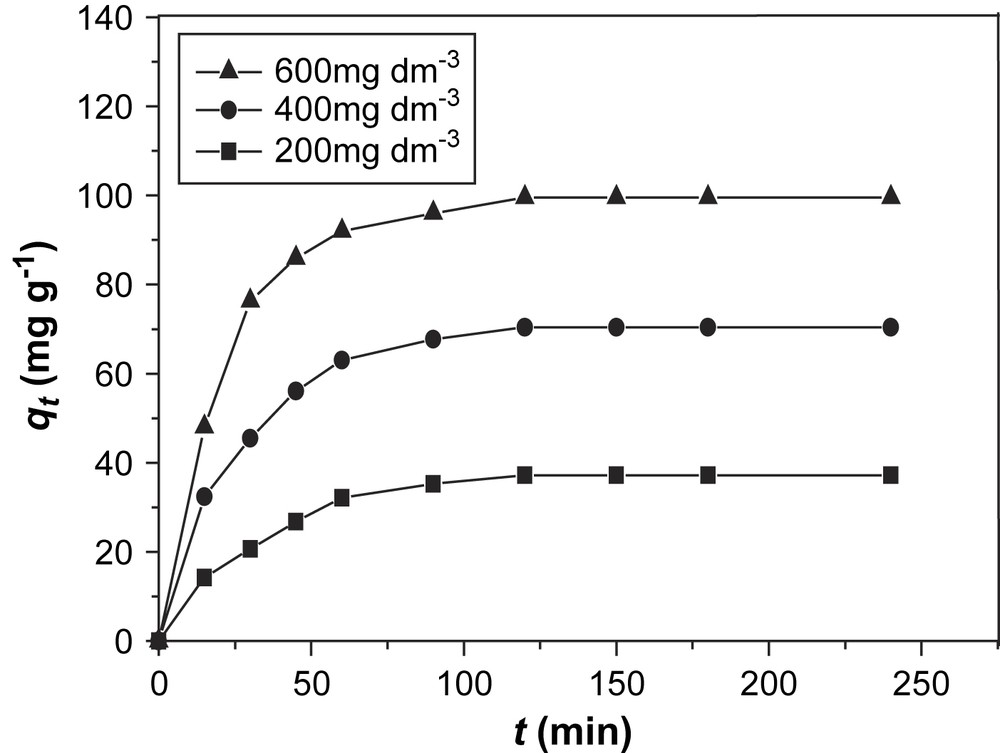
Effect of initial concentration on adsorption of BV14 on Na-bentonite.
The results show that the Na-bentonite is a more effective adsorbent than Ca-bentonite. In addition, the amount of adsorbed dye increased with the increase of initial concentration of the dye. Increasing the initial BV14 concentration increases the mass gradient between the solution and the adsorbent, and therefore, the rate at which BV14 molecules pass from the bulk solution to the particle surface and the amount of transfer at equilibrium.
3.1.2 Influence of contact time
Figs. 2 and 3 show that equilibrium is reached within roughly 90 min, independently of the adsorbent or the initial concentration of the dye, which indicates a relatively fast process.
3.1.3 Effect of salt addition
It is well known that salts have an effect on these types of adsorption processes [9]. In this work, BaCl2 and KCl were selected as model salts to investigate their influence on the adsorption of BV14 on bentonite. The results are presented in Figs. 4 and 5.
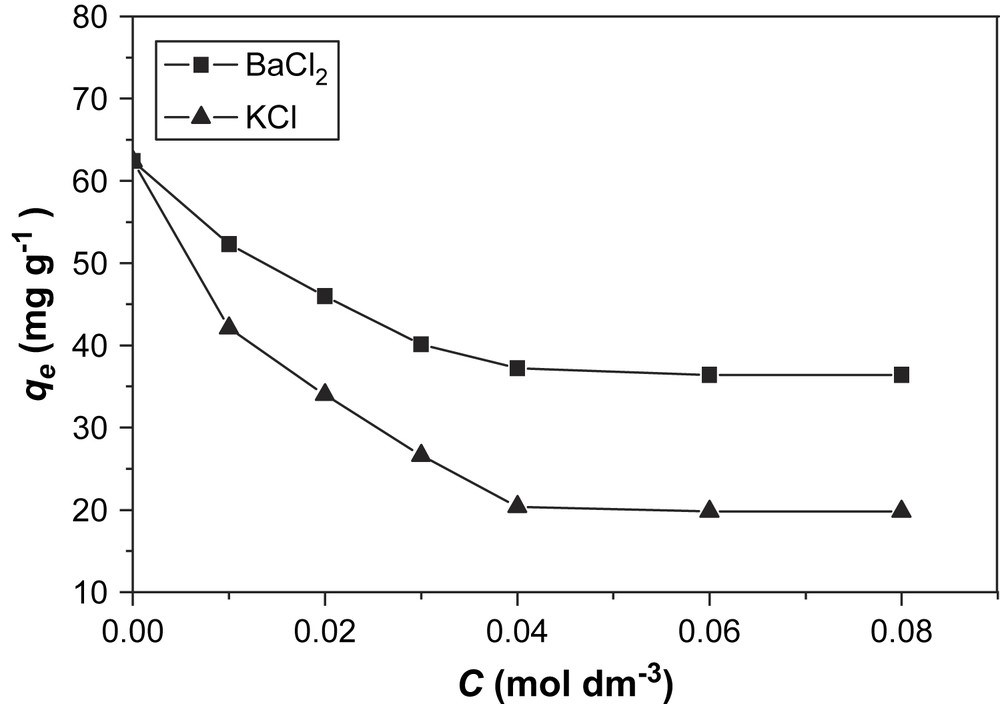
Effect of salt concentrations on the adsorption of BV14 on Ca-bentonite.
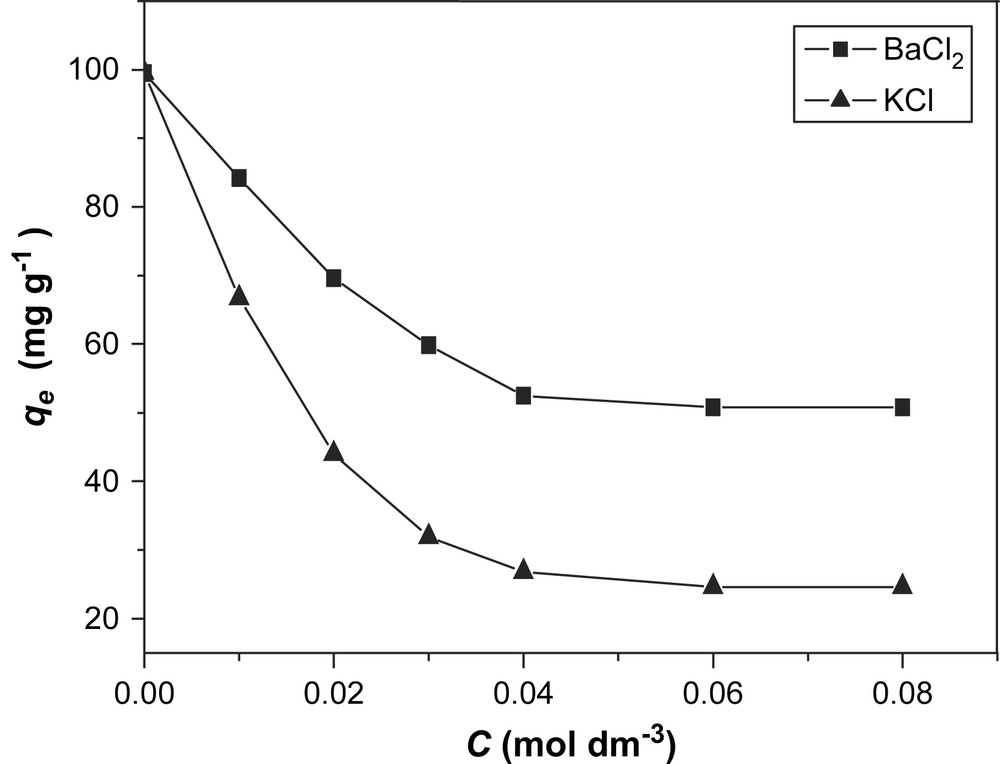
Effect of salt concentrations on the adsorption of BV14 on Na-bentonite.
It was observed that the presence of salts decreased the amount of dye adsorbed on bentonite at equilibrium (qe). Because the adsorption of basic dyes on bentonite is likely to be dominated by ion-exchange processes [10], the results may be explained in terms of the competition between BV14 and the cations in the added salt for the binding sites in the adsorbent.
3.2 Adsorption isotherm
In this work, the Langmuir model and the Freundlich model were used to describe the adsorption of BV14 on bentonite. The linear form of the Langmuir model can be represented by the following relation:
| (2) |
The linear form of the Freundlich isotherm model is given by the following equation:
| (3) |
The Langmuir and Freundlich constants were determined after fitting the data to the respective equations through linear regression analysis.
The Freundlich adsorption isotherms of BV14 on bentonite are shown in Fig. 6. The calculated values of the constants in Langmuir and Freundlich equations and the regression correlation coefficients (r2) are given in Table 2. It can be seen that both the Langmuir and Freundlich adsorption models were suitable for modeling the adsorption of BV14 on bentonite.
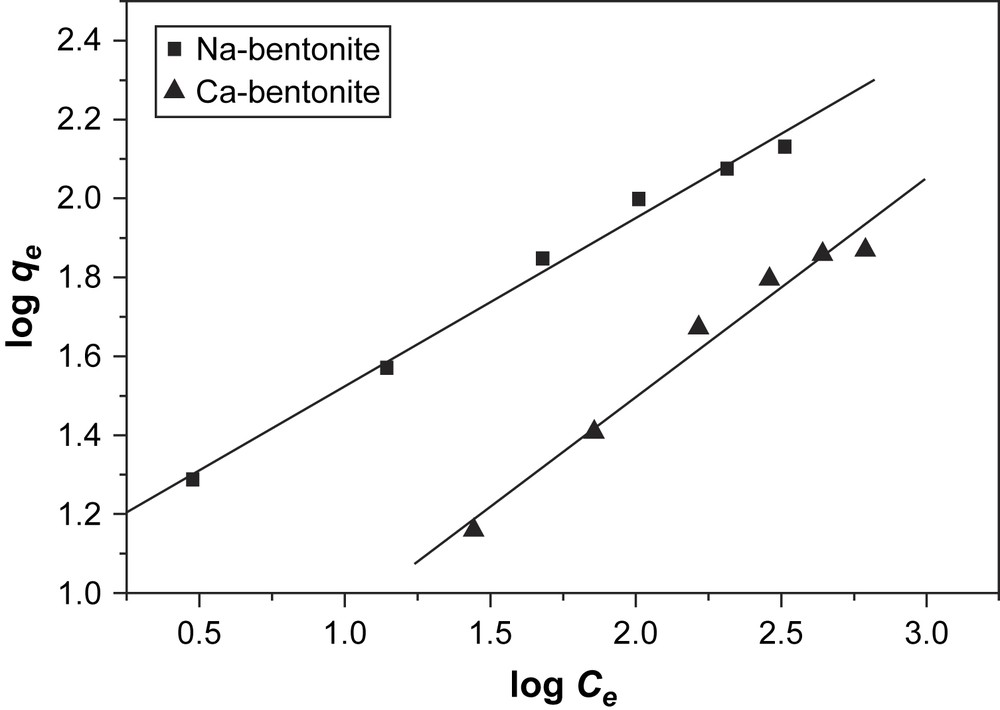
Freunlich isotherms for adsorption of BV14 on bentonite.
Values of the constants in Langmuir and Freundlich models
| Adsorbent | Langmuir | Freundlich | ||||
| Q0 (mg g−1) | b (dm3 mg−1) | r2 | n | K | r2 | |
| Na-bentonite | 147.49 | 0.0244 | 0.994 | 2.345 | 12.519 | 0.995 |
| Ca-bentonite | 100 | 0.00562 | 0.996 | 1.799 | 2.427 | 0.987 |
3.3 Kinetics of adsorption
Pseudo-first-order and pseudo-second-order kinetic model [11,12] were used to investigate the adsorption processes of BV14 on bentonite. The linear form of pseudo-first-order model is given by:
| (4) |
The linear form of pseudo-second-order model can be represented in the following form:
| (5) |
The results of fitting experimental data with the pseudo-first-order and pseudo-second-order models (Fig. 7) for adsorption of dye onto bentonite are presented in Table 3. As can be seen, the correlation coefficients (r2) is 0.999 for pseudo-second-order kinetic model and 0.956–0.963 for the pseudo-first-order model, indicating a better fit with the pseudo-second-order model. The better fit of this model to the experimental data is also seen in the much closer agreement between the experimental values for qe and those from the second-order model, when compared to the qe values generated from the first-order model.
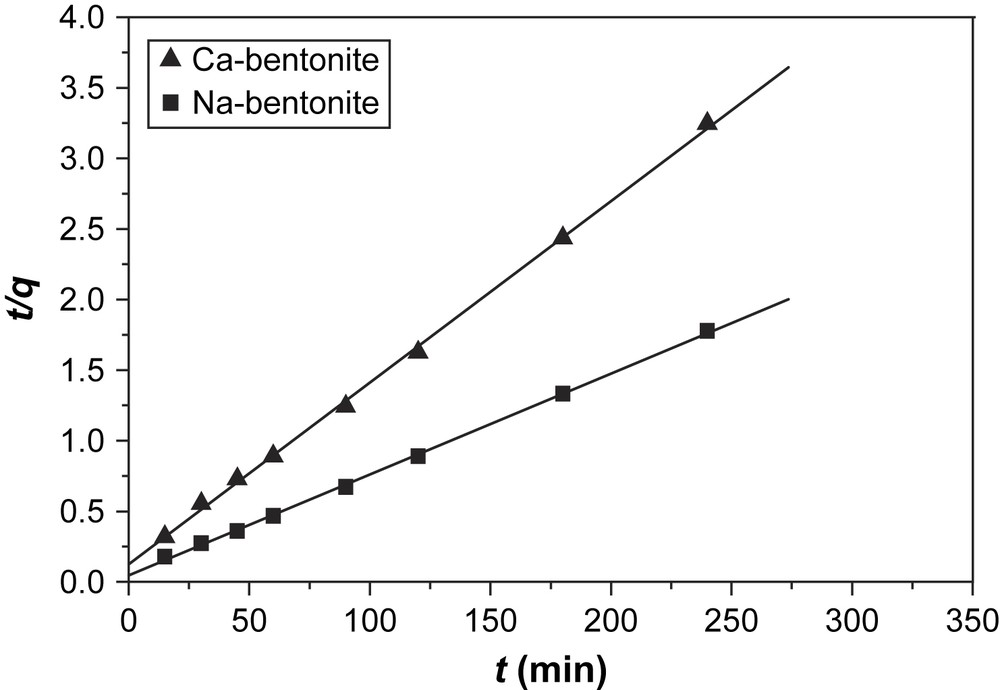
Second-order kinetic plots for the adsorption of BV14 onto bentonite.
Kinetic parameters for the adsorption BV14 onto bentonite
| Adsorbent | k1 (min−1) | qe (cal) (mg g−1) | r2 | k2 (g g−1min−1) | qe(cal) (dm3 mg−1) | r2 | qe (exp) |
| Na-bentonite | 7.57 × 10−2 | 300.4 | 0.956 | 1.08 × 10−3 | 139.9 | 0.999 | 135.0 |
| Ca-bentonite | 5.64 × 10−2 | 117.1 | 0.963 | 1.29 × 10−3 | 77.82 | 0.999 | 73.96 |
3.4 The changes of crystalline structure of bentonite during adsorption
X-ray diffraction analysis was performed to investigate the effects of BV14 adsorption on the basal spacing of bentonite. XRD patterns of Ca-bentonite and Na-bentonite before and after adsorption of BV14 are displayed in Fig. 8. It is noticeable that the adsorption of dye causes an increase in the basal spacing of bentonite. The results were likely due to the fact that organic cations insert themselves into the aluminosilicate sheets and expand the interlamellar spacing [13–15]. The change in the basal spacing of the bentonite structure resulting from the adsorption process is an indication of the extent to which the sorbate is reaching the interlamellar sorption sites [16]. The larger increase in basal spacing was found in Na-bentonite, implying a greater uptake on Na-bentonite than that on Ca-bentonite, which is consistent with the results in Figs. 2 and 3.
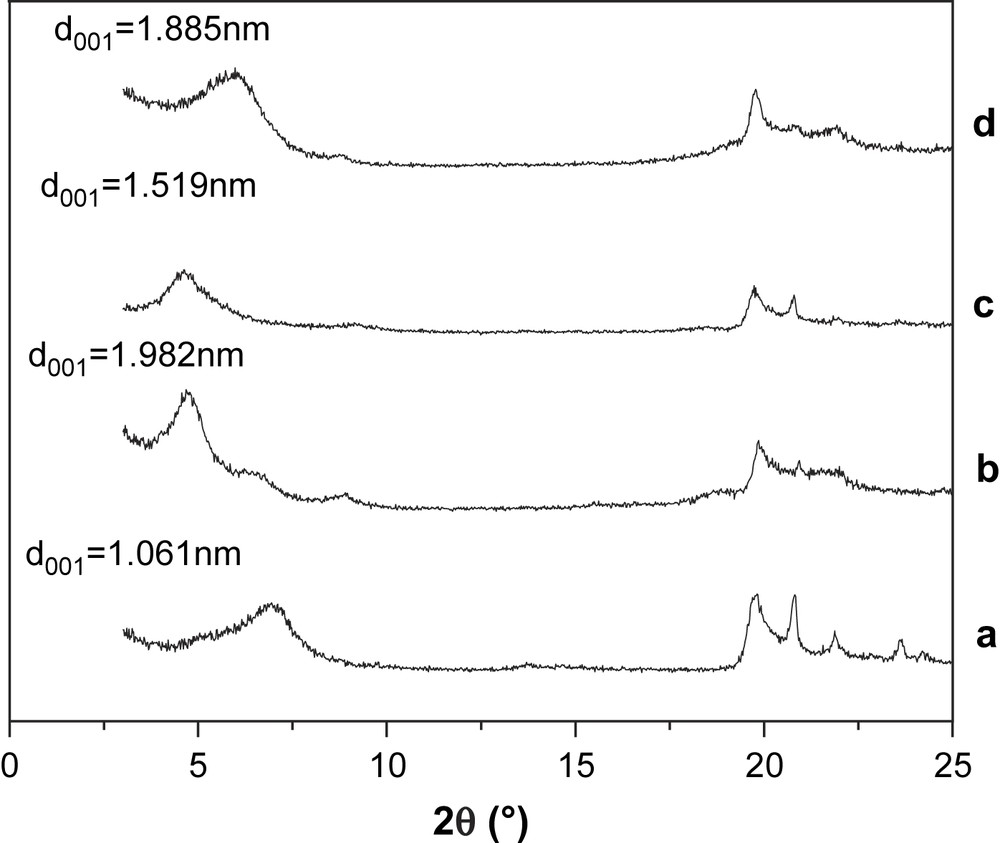
XRD patterns of various samples: (a) Na-bentonite; (b) Na-bentonite after adsorption of BV14; (c) Ca-bentonite; (d) Ca-bentonite after adsorption of BV14.
4 Conclusion
The adsorption isotherm and kinetics of BV14 from aqueous solutions on Ca-bentonite and Na-bentonite were studied in a batch experimental system. The results showed that the equilibrium adsorption is achieved in 90 min. The adsorption of BV14 increases with increasing initial BV14 concentration, and the adsorption process was influenced by the presence of salt, with a decrease in the adsorption of BV14 observed with increasing concentrations of BaCl2 or KCl. The greater adsorption capacity of Na-bentonite may be ascribed to its larger cation exchange capacity. The Langmuir and Freundlich isotherm adsorption models were found to be applicable for the experimental data. The pseudo-first-order and pseudo-second-order kinetic models were used to describe the kinetic data, with the latter model showing a better fit to the experimental data. X-ray diffraction of the crystalline structures of two kinds of bentonite before and after adsorption of BV14 showed evidence of an increase in the basal spacing of bentonite as a result of adsorption.
Acknowledgements
The authors are indebted to the Natural Science Foundation of Guangxi (Guikeji-0448085) for financial support and gratefully acknowledge the assistance of Visiting Professor Donald Barnes of Guangxi University for his helpful discussion and suggestions.


