1 Introduction
Water-stable room-temperature ionic liquids (RTILs) can be defined as salts with a melting point below 100 °C. They are composed of bulk organic cations such as 1,3-dialkylimidazolium or tetraalkylammonium, associated with various inorganic anions [1]. RTILs are considered as promising solvents for chemical processing due to their remarkable properties, including negligible vapor pressure, high thermal stability, and relatively good electrical conductivity. Recently it was shown that ionic liquids [BuMeIm]X, where BuMeIm+ is 1-butyl-3-methylimidazolium and X− is PF6− and (CF3SO2)2N− (Tf2N−), exhibit high stability with respect to γ-radiolysis [2], which makes them attractive for actinide chemistry. In particular, the substitution of RTILs for high-temperature molten salts could eliminate many of the technical and safety concerns involved in pyrochemical processes. Even water-immiscible RTILs are hygroscopic and usually contain some water. Knowledge of actinide behavior in ionic liquids in the presence of water is thus indispensable for any potential application. It was recently shown that An(IV) hexachloro complexes are stable with regard to hydrolysis in [BuMeIm]Tf2N [3,4]. However, the hydrolysis instability of PF6−-containing ionic liquids has often been noted in the literature [5,6]. Hydrolysis of PF6− forms a mixture of products, including HF, POF3, PO2F2− [7], etc., which can influence the behavior of actinides in PF6−-based RTILs. In this paper we report that AnCl62− complexes, where An(IV) is Th(IV), Np(IV) or Pu(IV), are able to accelerate PF6− anion hydrolysis in hydrated [BuMeIm]PF6, followed by the formation of soluble and insoluble species of An(IV).
2 Experimental
The ionic liquid [BuMeIm]PF6 was prepared by a metathesis reaction from [BuMeIm]Cl and HPF6, as recently described [2]. The RTIL was washed with deionized water (18 MΩ cm) to neutral reaction. The absence of chloride ions was verified by an AgNO3 test. Organic impurities were removed from water pre-equilibrated RTIL by mixing with activated carbon for 12 h, after which the mixture was passed through a column with small amounts of acidic alumina at the bottom of the column to ensure complete separation of carbon particles after filtration. The RTIL was dried in vacuo (∼5 mbar) at 80 °C for about 6 h. 1H NMR analysis revealed the absence (<0.1%) of impurities in the purified ionic liquid. The water concentration in the dried RTIL was found to be 0.038 M by Karl–Fisher coulometric titration. It should be noted that the ionic liquid adsorbs water during manipulations in contact with air up to a concentration about 0.1 M.
[BuMeIm]2[AnCl6] complexes, where An(IV) is Np(IV) or Pu(IV), were prepared by precipitation from the corresponding An(IV) solutions in HCl in the presence of [BuMeIm]Cl, as recently described [3]. The [BuMeIm]2[ThCl6] complex cannot be precipitated from concentrated HCl solutions, even with a large excess of [BuMeIm]Cl. This complex was prepared as follows: a known amount of ThO2 was dissolved in a minimal volume of concentrated HCl. [BuMeIm]Cl was then added to this solution at a Th(IV):[BuMeIm]Cl molar ratio of 1:2. The solution was evaporated under reduced pressure at room temperature to a solid white residue. HCl was removed from the outlet gas using a trap filled with granulated KOH. The precipitate was dried several days in a desiccator over silica gel. The resulting compound is readily soluble in the studied ionic liquid, in contrast with hydrated Th(IV) chloride prepared according to the same procedure, but without the addition of [BuMeIm]Cl. Unlike [BuMeIm]Cl, the Th(IV) complex is not hygroscopic. The prepared solid can therefore be assumed to be a [BuMeIm]2[ThCl6] complex and not a mixture of Th(IV) chloride and [BuMeIm]Cl. FTIR spectra of all the prepared complexes reveal the presence of the BuMeIm+ cation (ν(C–H) aromatic, s 3171, 3124 cm−1; ν(C–H) aliphatic, s 2966, 2939, 2878 cm−1; ν(ring), s sym 1575, 1467 cm−1; ν(MeC–H) asym 1431, 1386 cm−1; ν(ring), s sym 1170 cm−1). Water molecule vibration modes (ν1 and ν3, 3000–3800 cm−1; ν2, 1595–1650 cm−1) are observed only for the Th(IV) hexachloro complex, probably due to the presence of crystallization water, which was not completely removed during the drying procedure. [BuMeIm]2[AnCl6] complexes, where An(IV) is Np(IV) or Pu(IV), are anhydrous according to FTIR measurements.
Vis/NIR spectra were collected by a Shimadzu UV-3101 PC spectrophotometer using 1-cm quartz cells sealed airtight by Teflon stoppers. The diffuse solid-state reflectance spectra were measured with a U-3000 Hitachi device equipped with a 60-mm-diameter integrating sphere. FTIR spectra were collected by a Nicolet Magna-IR spectrometer in KCl pellets. NMR spectra were collected with a Unity Inova Varian 400 MHz spectrometer. For liquid-state samples two 5-mm probes were used: a 1H{13C/X} tunable triple resonance probe for 1H and all 2D heteronuclear correlation experiments, and an AutoSwitchable 5-mm probe for 19F, 13C and 31P 1D experiments. Concerning solid-state samples, two Cross Polarization/Magic Angle Spinning (CP/MAS) probes were used: a 7-mm VTN Bruker probe for radioactive samples, and a 5-mm Varian probe for classical powder materials. The 7-mm zirconia rotors were used with a hermetic Kel-f insert for all radioactive samples. A specially equipped glove box allowed safe handling of the Kel-f insert in the 7-mm ZrO2 rotor. 13C and 31P solid-state spectra were performed with TOSS pulse sequences.
3 Results and discussion
3.1 Vis/NIR spectroscopic study
All prepared An(IV) hexachloride complexes are readily soluble in [BuMeIm]PF6. The Vis/NIR absorption spectra of the freshly prepared solutions for Np(IV) and Pu(IV) shown in Fig. 1 fit well with the diffuse solid-state reflectance spectra of the corresponding solid complexes (see Supporting information). These spectra are also similar to the Vis/NIR spectra of Np(IV) and Pu(IV) hexachlorides in [BuMeIm]Tf2N ionic liquid [3]. The strong similarity of the solid and solution spectra suggests that AnCl62− is the largely predominant chemical form of actinide(IV) in [BuMeIm]PF6 solutions. However, unlike [BuMeIm]Tf2N solutions, the Vis/NIR spectra of Np(IV) and Pu(IV) hexachlorides are not stable in [BuMeIm]PF6 ionic liquid. Fig. 1 shows the evolution of these spectra during one week of storage in tightly closed 1-cm quartz cells. Moreover, the precipitation of solid products was observed for all studied complexes in approximately 10 days of storage. The precipitates were removed by centrifugation, washed twice with acetone and dried in air. The dry solids were white–yellow Th(IV), green Np(IV), and pink Pu(IV).
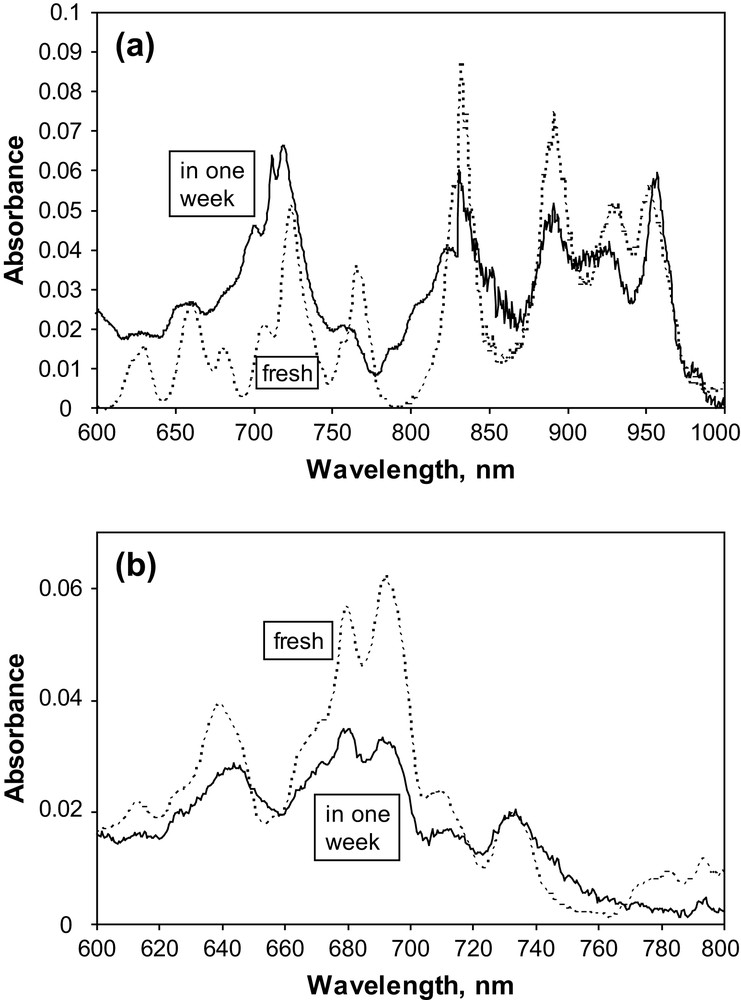
Vis/NIR spectra of: (a) 0.005 M [BuMeIm]2[NpCl6] in [BuMeIm]PF6; (b) 0.005 M [BuMeIm]2[PuCl6] in [BuMeIm]PF6.
3.2 Solid-state NMR study
The 13 C CP/MAS spectrum of the solid product for Th(IV) strongly resembles that of RTIL in DMSO-d6 solutions (see Supporting information) [1]. Table 1 shows the 13C chemical shift values for solid products with respect to those for RTIL solution.
The chemical shifts of 13C atoms, δ, in [BuMeIm]PF6 and Th(IV) solid products
| Carbon number in BuMeIm+ | [BuMeIm]PF6 (δ, ppm) | Th(IV) solids (δ, ppm) |
| 2 | 123.5 | 120 |
| 3 | 125 | 128 |
| 5 | 138 | 149 |
| 6 | 50 | 43 |
| 7 | 33 | 37 |
| 8 | 20 | 19 |
| 9 | 14 | 13 |
| 10 | 37 | 31 |
Fig. 2a demonstrates that the NMR MAS 31P spectrum of the solid product is very different from that of PF6− in DMSO-d6 (septuplet at 42.2 ppm), indicating the absence of the hexafluorophosphate anion in the solid products. The 31P MAS NMR spectrum of solids reveals the presence of two species with PO bonds according to the observed chemical shifts at −9 and −18 ppm, respectively. The multiplet with the relative peak intensities equal to 1:4:6:4:1 and the coupling constant 1JPF = 711 Hz could be assigned to some phosphorous species with P–F bonds. The 19F MAS NMR spectrum shown in Fig. 2b exhibits two doublets (roughly separated by 120 Hz) with the same coupling constant 1JPF, as for the 31P nucleus. Two F–P environments are then observed, but other NMR experiments should be done for further assignments. Surprisingly, the 19F MAS NMR spectrum gives no indications on ThF4 formation. The recently published spectrum of this compound is rather complex and is made of different group of bands with large chemical shift anisotropy and δ 19F values in the range of 53–101 ppm [8]. Such kind of spectrum is not observed for the solids precipitated from Th(IV) solution in [BuMeIm]PF6.

(a) 31P NMR of the solids precipitated from [ThCl62−] solutions in [BuMeIm]PF6 (inset: 31P NMR spectra of [BuMeIm]PF6 in DMSO-d6 solutions). (b). NMR 19F of the solids precipitated from [ThCl62−] solutions in [BuMeIm]PF6.
Briefly, the NMR studies reveal the formation of several products of PF6− anion hydrolysis which are able to precipitate An(IV) from [BuMeIm]PF6. Formation of the PO2F2− anion as one of the intermediates can be presumed by similarity with PF6− hydrolysis catalyzed by Ag(I) [7]. However, additional investigation is needed to establish the composition of these products.
3.3 FTIR spectroscopic study
The FTIR spectra of the solid products for Th(IV) and Pu(IV) are shown in Fig. 3. The presence of C–H and C–C vibrations (Fig. 3a) confirms that the solids contain the BuMeIm+ cation. Moreover, the FTIR spectra of both products clearly indicate the presence of PO stretch vibration modes at 1147 cm−1 (Th) and 1093 cm−1 (Pu), which can be related to phosphate or fluorophosphate coordinated to An(IV) cations. These vibrations were observed neither in pure RTIL nor in initial An(IV) hexachloro complexes (Fig. 3a). The νas(PO) IR band for thorium products is shifted to higher frequency if compared with thorium phosphate-hydrogenophosphate (1010–1125 cm−1) [9]. That can be related to weaker binding of fluorophosphates to Th(IV) cation than that of phosphates. The relative shift in νas(PO) for Th(IV) and Pu(IV) is difficult to interpret since the solids are composed of the mixture of products originated from PF6− anion hydrolysis and the product ratio can differ for thorium and plutonium. The strong P–F stretch vibrations at 854 cm−1 (Th) and 843 cm−1 (Pu) are close to those of pure RTIL (837 cm−1) and of the fluorophosphate anion PO2F2− (847 cm−1) [7]. The observed P–F vibrations cannot be related to the PF6− anion according to NMR data. Thus FTIR measurements suggest that the solids precipitated from [BuMeIm]PF6 most probably consist of a mixture of actinide(IV) phosphates and fluorophosphates.
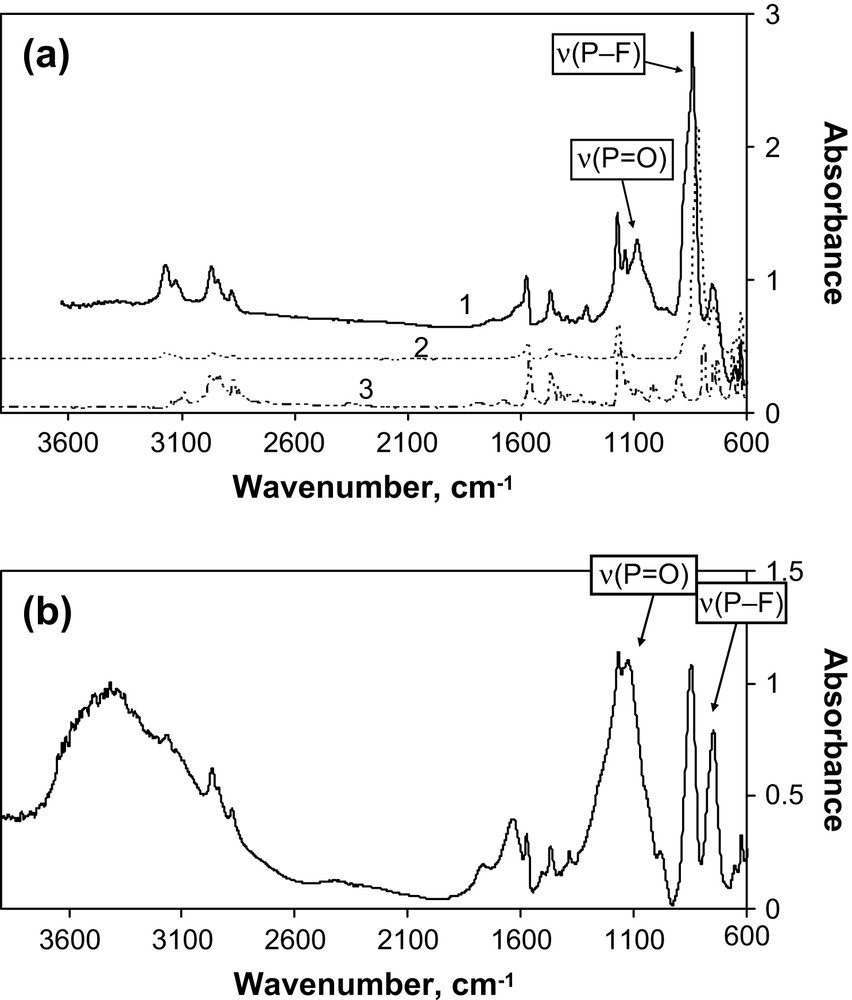
(a). FTIR spectra of the solids precipitated from [PuCl62−] solutions in [BuMeIm]PF6 (1), liquid [BuMeIm]PF6 (2) and solid [BuMeIm]Cl (3). (b). FTIR spectrum of the solids precipitated from [ThCl62−] solutions in [BuMeIm]PF6.
3.4 Probable mechanism of PF6− anion hydrolysis
The NMR test of freshly prepared pure [BuMeIm]PF6 indicates the absence of PF6− anion hydrolysis products for at least several weeks, indicating that AnCl62− anionic complexes are able to accelerate PF6− anion hydrolysis, similarly to the known catalytic effect of acids [5] and d-transition metal cations [7,10] on PF6− anion hydrolysis. The probable mechanism of PF6− anion hydrolysis with AnCl62− complexes could include the exchange between a water molecule from the ionic liquid and a chloride anion from the complex:
| AnCl62− + H2O ⇌ AnCl5(H2O)− + Cl− |
| AnCl5(H2O)− + PF6− ⇌ AnCl5− + HF + F5P(OH)− |
| AnCl5− + Cl− → AnCl62− |
| AnCl62− + nF− → AnCl6−nFn2− + nCl− |
4 Conclusions
The instability of AnCl62− complexes in a hydrated room-temperature ionic liquid is related to the hydrolysis of the PF6− anion accelerated by AnCl62− complexes.
Fluorophosphates and phosphates formed from the PF6− anion cause the precipitation of An(IV) from RTIL solutions.
Acknowledgements
We are grateful to Dr. M.-C. Charbonnel for the aid in FTIR studies, and C. Gimenez for the NMR measurements. This work was supported by DEN/DDIN/DPSF/CCC and DEN/DSOE/RB (CEA, France).
Appendix Supporting information
Solid-state Vis/NIR reflectance spectra of [BuMeIm]2[NpCl6], [BuMeIm]2[PuCl6], and 13C NMR spectra of the solids precipitated from [ThCl62−] solutions in [BuMeIm]PF6.


