1 Foreword
The last time I (PB) met Pierre Gilles was in Florence. He was invited for a conference and to deliver the Primo Levi award of the Italian Chemical Society to distinguished young scientists. We had the congress dinner in the charming atmosphere of the courtyard of Palazzo Pitti together with Françoise and my wife, and we mainly discussed art and restoration. He was interested in my “side” scientific activity in conservation of Cultural Heritage where we transferred Soft Matter to the conservation of works of art and, in particular, to consolidation of paintings, deacidification of paper, canvas and wood. He was curious and enthusiastic like a child and as a good scientist should be. I really enjoyed, as usual, talking with him. Sometime one learns more Science in a dinner than reading hundreds of papers. This contribution, that is quite untypical for its structure and contents, is our tribute to the scientific and artistic side of Pierre Gilles.
2 Introduction
The study of the properties and the synthesis of nanostructured materials is considered one of the most important research fields for the forthcoming decades. Research on nanoscience consistently developed starting from the eighties and more than 50,000 articles have been published in 2007 containing the “nano” prefix. According to the United States National Science Foundation, the governments of the most advanced countries invested in nanotechnology more than 4.1 billions of US-dollars during the last year, progressing from the 1.5 billions in 2001 and 432 millions in 1997 [1]. Nanotechnologies are usually considered to be principally involved in electronics and in the miniaturization of systems, but they are pervasive and contribute to different fields, such as chemistry, biology and physics.
Nanostructures are constituted by clusters of a few thousands of atoms, and show properties that are intermediate between the molecular and the bulk matter state. This is due to the high area/volume ratio of the clusters that strongly affects the physico-chemical behavior of these systems. Advantages obtained from the nanostructure of materials include low sintering temperature, super-plasticity effects, increase in diffusivity, improving of the dielectric and tribologic properties. This enables the production of better quality ceramic materials, soft (“low-loss”) magnetic materials, and materials with high ferroelectric and piezoelectric properties. Nano-structuration confers high hardness, better wear resistance and high density to materials that require an ultrahigh-strength [2]. The high specific surface may give the explanation for the catalytic activity and for the high gas absorbance by finely divided nanostructured materials.
It is easy to understand that research of new materials and nanostructured devices is a central focus in soft and hard materials science. The production of innovative materials starting from nanosized precursors is offering huge opportunities for industrial, biomedical and environmental applications, which were inconceivable just a few years ago. The main targets include the synthesis of crystalline rather than amorphous products (or in some cases amorphous rather than crystalline), lower size heterogeneity, and improved purity and stability of the final products.
Many synthetic procedures were developed in order to obtain high yields and to control the average dimension of particles and the polydispersity [3]. The success of synthetic procedures is reduced by the tendency of the particles to reorganize in larger agglomerated structures. This is a limitation to the great potential applications of these systems to different fields, including the world cultural heritage conservation.
Applications of nanotechnology to wall paintings' conservation have recently provided clear evidences of the huge potentiality of this emerging Science for the conservation of Cultural Heritage [4]. Humble nanoparticles of calcium hydroxide [5–7] and nano-magnetic gels embedded with microemulsions have been recently used to restore frescoes [8], microemulsions have been formulated to remove polymers or clean frescoes and oil paintings [9–11], and nanoparticles of calcium and magnesium hydroxide have been used to de-acidify paper, wood and canvas [12–16].
3 Chemistry contribution to conservation science
The peculiarity of the research in Cultural Heritage, where basic studies are usually associated to applied research and restoration workshops, resides in its multidisciplinarity. The co-operation with conservators, private and public institutions for diagnostics, and experimentation of innovative methodologies is fundamental in the definition of the restoration procedures for the conservation of the works of art.
During the last twenty years, the contribution of scientists to this field has radically grown up. The knowledge of a work of art is no longer restricted to humanists; new restoration tools have been borrowed from Materials Science and Soft Condensed Matter, and the contribution of chemists and physicists is becoming predominant for the prediction of the degradation insight and for the rescue of Cultural Heritage.
Chemical “corrosion”, which induces the powdering of paintings, and the mechanical action (mainly from rain, wind and dust) are the main factors responsible for the weakening of the porous structure and surface layer of the materials used in cultural heritage [17]. Nowadays, thanks to the contribution of Chemistry we know how to address most of the degradations due to chemical “corrosion” and the restoration provides plenty of benefits to works of art. Most interventions allow the reinforcement of the porous structure and the consolidation of the surface layer of the work of art (that is usually the most precious). Protective treatments, after cleaning and consolidation, confer longer lifetime for the works of art. Restoration strives the opacity of the surface by minimizing the light scattering effects, and the protection of the surfaces strongly reduces the degradation from pollutants and water condensation.
All the interventions directed to preservation of the historical meanings of the works of art are commonly designed as Conservation treatments. Conservation is, for example, the cleaning procedures, the shooting by a camera for the preservation of the historical memory, the making-up of lightening systems in a museum, the strict control of the visitors' flux in a site, and so on. In a strict sense, all the chemical treatments to preserve works of art from damage and deterioration are the matter of conservators and scientists aiming to rescue the original materials. On the other hand, despite a common opinion, every restoration treatment should be considered as invasive. Conservators often have to remove chemicals from the works of art surfaces, such as fat, salts, varnishes, pollutants; this is, usually, done by chemical and mechanical methods, which may also affect the substrate. Moreover, chemicals for consolidation and protection are used for improving the physico-chemical and mechanical features of the materials. These materials are introduced within the porous structure of the works of art, strongly modifying the original characteristics of the objects, making very hard the prediction of the lifetime of the restored materials.
Some basic principles have been established, and accepted, by most conservators, in order to reach the best results in a restoration workshop [17]. Basically, three points can be stressed:
- 1 the treatment should be reversible so that at any time it should be possible to revert to the status before the treatment;
- 2 all the applied chemicals should ensure the maximum durability and the chemical inertness;
- 3 the chemicals used in the conservation treatment should be compatible with the substrate.
However, scientists know that reversibility does not exist [18]. It follows that methods based on compatible materials should be preferred, so that the original features of the materials are slightly changed, or, at the best, they are not changed at all.
Modern Conservation Science originated from the tragic flood that devastrated Florence and Venice in 1966, imposing the search for new conservation methods [19]. At the International ICOM Conference in Amsterdam (1969) a new method for in situ consolidation of wall paintings was proposed by Enzo Ferroni, from University of Florence [20], and the conservator Dino Dini. The method known as “barium or Ferroni–Dini method” was the first example of a chemical and structural consolidation technique for the restoration of wall paintings that underwent phenomena of salts' crystallization and/or sulphatation.
Since that time, our research activity in the conservation field has been focused on the development of innovative methods using materials compatible with the “work of art”; for example inorganic materials should be used, when possible, to restore the inorganic constituents of the works of art.”
The Ferroni–Dini method represents a neat example of the philosophy beneath the Science of Conservation, and paved the way for the development of some of the most advanced and best performing conservation techniques.
The Ferroni–Dini method inverts the chemical reactions that produce the degradation of wall paintings, stabilizing the structure of the mortar and regenerating the binder of the painted layer, that is calcium carbonate. The method is based on the application of ammonium carbonate and barium hydroxide solutions on the painting surface. The treatment eliminates harmful gypsum (formed by environmental pollution) and promotes the formation of new slaked lime (Ca(OH)2), formed in situ, which acts as the fresh binder, giving new setting for mortar with maximum physico-chemical compatibility. The “barium” method is at the moment one of the more powerful techniques available for frescoe consolidation; its success depends on the salts' removal and, at the same time, on the reinforcement of the porous structure of the fresco painting.
A few years later, when the extraordinary restoration performed on the wall paintings by Beato Angelico (San Marco, Florence), where the Ferroni–Dini method was used for the first time, Ferroni and Dini realized that the consolidation treatment was performing much better with time. This finding convinced Ferroni that a large excess of barium hydroxide used during the application could be responsible for the slow and progressive transformation of calcium carbonate to calcium hydroxide, i.e. barium hydroxide generated new binder from the calcium carbonate. The newly formed binder was considered to be responsible for the good consolidation, similar to a new setting process of lime (calcium hydroxide). Later, we proposed to apply calcium hydroxide in the form of nanoparticles for the consolidation of wall paintings. These nanoparticles dispersions, applied to the conservation of Cultural Heritage in several countries, are the most powerful tool for the consolidation of wall paintings and limestones.
4 The “French” microemulsion
Restoration of wall paintings requires several complex operations: among these, and very important, is the painting cleaning. In the middle of the eighties our group was interested in the removal of hydrophobic impurities from the paintings by Masaccio and Lippi in the Brancacci Chapel in Florence, considered one of the most important masterpieces of the Renaissance period. UV fluorescence investigations and preliminary cleaning tests revealed the presence of a patina from wax spots due to extinction of altar votive candles. This unexpected contamination required a great effort to remove this hydrophobic material deposited and partially adsorbed within the heterogeneous porous and hydrophilic structure of the wall.
Organic solvents were commonly used in this case in order to solubilize the wax impurities; however, these solvents promoted the diffusion of solubilized wax inside the wall. The spreading of the wax lowered the optical density of the patina, producing an apparently a “good looking effect” to the naked eye. A more detailed investigation with UV-light showed that the impregnation of the porous structure of the wall that consistently altered the physico-chemical characteristics of the substrate.
This prompted us to study new water–oil–surfactant systems to solubilize and extract wax from the porous matrix of the wall. The paper from Pierre Gilles De Gennes and Christiane Taupin about microemulsions inspired the first microemulsion system, tailored for cleaning of works of art surfaces [21].
Due to the thermodynamic stability and the detergency efficacy, oil-in-water microemulsions are the best candidate for a successful cleaning of wax spots from fresco painting. The microemulsion used in Brancacci's paintings was very similar to the so called “French microemulsion” and was formed by dodecane nanodroplets, dispersed in an aqueous solution of sodium dodecylsulphate (surfactant) and pentanol (cosurfactant) [21–23]. The oil-in-water microemulsion ensured very low aggressiveness to the painted layer, due to the presence of water as a dispersing medium that remained in direct contact with the hydrophilic surface of the wall painting. Furthermore, water favoured the wetting of the wall and ensured the right contact time with the hydrophobic material to be removed. The microemulsion system was applied on the surface by proper equipment that ensured a continuous microemulsion flux by means of a pipet connected to a reservoir tank. Excellent results were obtained by this innovative approach, with a minimal disturbance for the work of art due to the restoration procedure (see Fig. 1).

Masaccio's wall paintings in Brancacci chapel, Florence: pre- (left) and post-removal (right) of wax spots.
This methodology has been transferred to the removal of polymers applied to works of arts during conservation interventions. Conservation treatments performed using synthetic polymers have produced severe degradation of the surface of the works of art, as clearly evidenced by the poor conservation conditions shown by several masterpieces in different countries. The removal of polymers is usually difficult since pure solvents are poorly effective due to the loss of solubility of the degraded polymers, as a consequence of the polymer oxidation and cross-linking reactions that, in addition, modify the characteristics of the painted surface.
The chemistry of surface-active agents provides several ways to remove polymers coating from painted surfaces (see Fig. 2). We formulated some nano-compartimentalized systems (micelle and microemulsions) specifically tailored for the removal of mainly paraloid B72 (ethyl-methacrylate methyl-acrylate copolymer), primal AC33 (ethyl-acrylate methyl-methacrylate copolymer), and mowilith DM5 (vinyl-acetate n-butyl-acrylate copolymer) resins, used as protection for painted surfaces.

Wall paintings in San Salvador church in Venice: pre- (left) and post-removal (right) of Paraloid B72.
The “oil” phase works as a nano-container where polymers can be solubilized and removed from the surface. The detergency depends on the huge surface of micelles or microemulsion nanodroplets where polymers can be solubilized. These systems enhance the polymer removal from surfaces and from the porous structure of works of art. The formulation and application of these systems would not be possible without the cultural framework generated by Pierre Gilles.
5 Nanotechnology to preserve Mesoamerican treasures
Mexican cultural heritage, which includes wall paintings from pre-Columbian to modern times, is one of the richest of the world and is an essential source of iconographic and historical information of Aztec and Maya civilizations, hence the interest in its conservation. Mesoamerican wall paintings' conservation is challenging for conservators and scientists [24]. The specific environmental conditions of sub-tropical areas pushed toward a revision of the general principles of conservation methodologies [25]. Professional conservation in Mexico dates back to 1960s and was mainly developed with the support of UNESCO. The influence of European Schools of Conservation, especially in the field of wall paintings, was fundamental for the training of the early Mexican conservators. Traditional conservation methods and materials were “imported” from the “European school” for the conservation of pre-Columbian works of art. However, the environmental conditions of Mexico, very different from those of Europe, forced Mexican conservators to modify those methods in order to find restoration procedures appropriate to the materials used for the Mexican works of art, and for the different climate, particularly the very different humidity. For example, Mexican conservators recognized well ahead of the Europeans that consolidation of paintings and stones with organic materials produced, in the Mexican climate, severe damage to the works of art. This is one of the reasons why most of Mexican restorers largely used lime-water, to avoid the use of organic materials for the consolidation of painted layers.
Synthetic acrylic or vinylic polymers, such as Paraloid B72, Mowilith DM5, and Primal AC 33, have been largely used for decades, producing devastating and completely unexpected degradation processes [24,26,27]. In controlled environments, such as those offered by colonial buildings, the results were often acceptable. On the other hand, at the archaeological sites of Palenque, Cacaxtla and Kohunlich, where many polymers were applied as fixatives, secondary effects were observed, e.g. the detachment and flaking of surfaces and a consistent acceleration of the chemical reactions involved in paintings' degradation. This is particular evident in Cacaxtla where wall paintings were consolidated by using the two consolidation methods available at that time: the Ferroni–Dini and polymers (mainly Paraloid). The paintings treated with the Ferroni–Dini method (inorganic method) are in good “health” while the other wall paintings treated with Paraloid are in a severe status of degradation, and without an immediate restoration they will be illegible in very short time. Similar degradation processes are present in the European works of art, but the degradation kinetics is much slower and apparently the works of art seem to be in a good state.
Unfortunately, the removal of degraded synthetic polymers is not easy and in some cases seems not possible at all [28]. With time, polymers become insoluble and chemical transformations (basically, oxidation reactions) modify the physico-mechanical properties of the materials. These processes affect the features of the substrate, e.g. the permeation to vapour of the surfaces is strongly reduced, sometimes colouring effects can be detected, and the shrinkage of the polymer film coating the surface induces de-cohesion and the detachment of the painted layer [29]. The application of chemicals similar to the original constituents of the works of art strongly reduces the risks due to the low compatibility among materials.
Present criteria for treatments, such as compatibility, minimal intervention or reversibility, have found only in the last years some practical applications with the emerging of new techniques based on nanotechnologies.
In the archaeological site of Calakmul (Mexico), a large number of wall paintings have been recently discovered. The paintings were buried by the ancient Maya population and, after their recovery, have been exposed to weathering and corrosion. The need for innovative conservation methods thus became apparent, following criteria of physico-chemical compatibility with the original materials, mainly inorganic. Therefore, we used only inorganic materials for the conservation treatments, and when possible we reverted the chemical reactions leading to the degradation of the works of art.
Calakmul, declared as a UNESCO world heritage site, is situated in the region of Peten, in the southern Campeche State (Mexico), close to the border of Guatemala [30,31]. The buildings are located in the Calakmul Reserve, a protected area of about 724,000 ha of tropical jungle. Calakmul was one of the most important centers of the classic Maya period (250–800 A.D.). In the site a large number of sculpted monuments can be found, as well as more than 120 stelae that report the history of the population that inhabited the city. The buildings are characterized by artistic and architectural complexities that highlight the extraordinary importance of the site. Evidence points out that the city was inhabited for more than 12 centuries, since 400 B.C. (Pre-Classic period), reaching its maximum development between 600 and 800 A.D. (Late-Classic period), and then being gradually abandoned around 900 A.D. (Post-Classic period). Calakmul was discovered in 1931 by the botanic Cyrus Lundell, who gave the site its present name that in Maya language means Ca ‘two’, lak ‘close’, mul ‘artificial hills’. The urban area of Calakmul extends for about 25 km2, and it is divided into six main areas: Gran Plaza, Gran Acròpolis, Acròpolis Norte o Acropolis Chik Naab, Grupo Nor-este, Grupo Sur-oeste and Pequena Acròpolis. These include about 6250 buildings, sculptures and monuments such as stelae and altars. Fig. 3 reports a view of the Gran Plaza from the top of structure II.

Calakmul (Mexico): views from the top of pyramid II.
The excavation work on the whole area has been planned and carried out since 1993 (Proyecto Arqueòlogico Calakmul). The project, supported by the government of the State of Campeche and by Instituto Nacional de Antropologìa e Historia (INAH), directed by the archaeologist Ramòn Carrasco Vargas brings together the different institutions, including the Universidad Nacional Autonoma de Mexico (UNAM) and CSGI-University of Florence, and involves archaeologists, conservators, anthropologists, architects, engineers, chemists and epigraphists.
Calakmul area is characterized by a sub-tropical climate with stable temperatures (25–35 °C) and high relative humidity from 75 to 95%. Such conditions accelerate the aging and the degradation of the synthetic polymeric materials that cannot be used for the conservation.
Fig. 4 reports two pictures of the structure “Group 6”, in Acròpolis Chik naab. Pictures on the left show the external side of the structure, while on the right it is shown the interior, characterized by the presence of banqueta, an elevated sidewalk with an extensive pictorial decoration.

Calakmul (Mexico): external (left) and internal view (right) of structure “Group A6”.
The paintings of Group 6 required, after their excavation, some consolidation treatments. In fact they showed deterioration phenomena, mainly due to water infiltrations and to the presence of salt solutions inside the porous matrix of the wall. The natural aging caused the detachment of the painted layer, the powdering of the surface, and a lost of cohesion between pigments and substrate.
As discussed above, the use of acrylic or vinyl polymers for the consolidation of painted surface would bring to the loss of the remained parts of the paintings, caused by the physico-chemical incompatibility between the polymers and the painted layer. Hence, a complete compatibility between the original materials and restoration products can be achieved by using calcium hydroxide. This is, in fact, the best way to consolidate wall paintings, since calcium hydroxide is the original binder used by artists. Lime-water could be used, but calcium hydroxide is scarcely soluble in water (1.6 g L−1). Stable dispersions of calcium hydroxide nanoparticles in non-aqueous solvents can be used to this purpose. The application of nanoparticles can be compared to the application of a very concentrated lime-water solution and overcomes the limit given by the poor solubility of calcium hydroxide in water.
Synthesis of calcium hydroxide nanoparticles, used for interventions on paintings of Group 6 in Acròpolis Chik Naab, is reported in the literature [32]. The nanoparticles dispersion was applied by brushing up to saturation on a small portion of wall painting surface, protected with Japanese paper (see Fig. 5). Some hours after application a de-mineralized water compress was put for about 1 h on the treated surface in order to keep it humid and to slow down the carbonation process. After the application no aesthetic drawback on the surface (whitening or chromatic alteration) was noticed. The sample was left to dry for 10 days before further analyses on it were performed.

Application by brushing a calcium hydroxide nanoparticles dispersion over a painted surface protected with Japanese paper (left); the same surface maintained wet with a water-filled cellulose compress (center); the painted surface at the end of the consolidation treatment (right).
The particles penetrate in the first layers of the painted surface, up to an average depth of 200–300 μm or more, and, once the alcohol is evaporated, turn into calcium carbonate. In this way the original binder for pigments is re-created in situ, and the consolidation is achieved.
Fig. 6 reports the particle size distribution of Ca(OH)2 nanoparticles used in Calakmul, obtained from the analysis of more than 300 particles. The log-normal distribution is centred at about 220 nm. Nanoparticles with dimensions from 150 to 280 nm behave very well for paintings consolidation; in fact they give a very high kinetic stability due to the tiny dimensions and are much smaller than the average porosity of plaster (and also of several limestones).
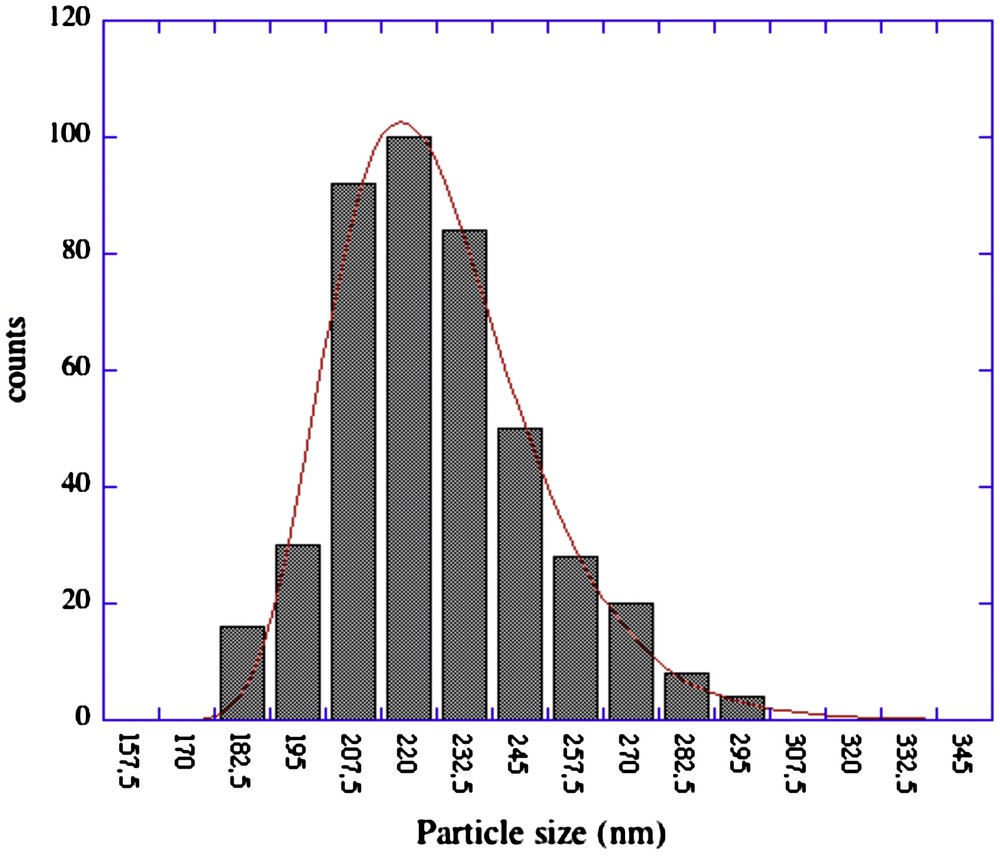
Particle size distribution of Ca(OH)2 nanoparticles obtained from the analysis of several TEM pictures.
In order to obtain more information about the consolidation effects of nanoparticle dispersions on painted plasters, several investigations were first performed in the laboratory. Some plaster model systems, made by using different lime/sand mixtures, were used. In particular, lime/sand ratios with a very low amount of binder (1:8 and 1:10 lime:sand v/v) were selected for testing. Mineral pigments were also applied with a “fresco” technique to reproduce a degraded painted surface. The model systems were treated with nanoparticles dispersions in order to re-build the mechanical properties of the plasters.
Table 1 reports some meaningful parameters to characterize the main properties of the untreated and treated plaster surfaces. Water sorption by capillarity and permeability to vapour was also determined since they are strictly connected to the porosity features of the treated surface. The comparison of the treated systems with a 1:2 lime/sand ratio plaster, which is typical of a well-preserved fresco, showed that nanoparticles' application reduces the capillary sorption of water and the plaster transpiration. Microdurimetry analysis determined the resistance of the surface to mechanical stress due to indentation and scratching and allowed estimating the hardness of the surface. After treatment the resistance of the treated surface was doubled. Powdering of the surface was also estimated by scotch tape test (STT) and the loss of pigments was strongly reduced after the consolidation by nanoparticles dispersions.
Evaluation of nanoparticles' treatment by measurements of physico-chemical and mechanical properties of painted model-plasters
| Lime:sand (v/v) | Capillary absorption coefficient [(g/cm2)s−1/2] | Permeability to vapour (g/m2 in 24 h) | Scratch-width by micro-durimetry (mm) | STT, scotch tape test (mg/cm2) |
| 1:2 | 0.56 | 595 | 1.0 | 0.28 |
| 1:8 untreated | 0.64 | 995 | 3.2 | 5.19 |
| 1:8 treated | 0.35 | 850 | 1.8 | 1.37 |
| 1:10 untreated | 0.72 | 987 | 4.4 | 7.12 |
| 1:10 treated | 0.42 | 717 | 2.4 | 4.12 |
Nowadays, application of nanoparticles (see Figs. 7 and 8) is in progress in some sub-tropical areas in Mexico [33]. This treatment protects the paintings from degradation consolidating the pigments and allowing the transfer to future generations of this unique cultural patrimony. Most of the methods we devised during the years come from the application of Soft Matter to Conservation. Without the De Gennes contribution to Soft Matter this would not be possible.

Different steps of an in situ application of nanoparticles dispersions (credits by Alice Desprat).

Maya wall paintings recently discovered inside structure 1 in Chick-Naab's acropolis.


