1 Introduction
Tin sulphide compounds SnxSy present physicochemical properties, optical quality, and electronic characteristics (bandgap varying between 1 and 2.3 eV depending on x and y values, high charge carrier mobility, high radiation strength…) adapted to the fabrication of optoelectronic devices such as solar cells [1–13]. SnxSy thin films can be prepared by different methods [1–4,13] among which the Chemical Bath Deposition (CBD) technique in a basic solution. However, basic solutions carry an unwanted precipitate [14]. Therefore we have chosen an acid solution to prepare SnxSy films by CBD and we have characterized the crystallography, the morphology, and the chemical properties of the obtained layers by using X-ray Diffraction (XRD), Atomic Force Microscopy (AFM), Energy Dispersive X-ray Spectroscopy (EDS), and Auger Electron Spectroscopy (AES). Results exhibit the role of the substrate nature, the deposition time, and the ratio between tin and thioacetamide (TA) concentrations [Sn]/[TA], on the layer properties.
2 Experimental procedure
Thin films are grown on glass and on SnO2/glass substrates for 60 min. Glass substrates are more particularly devoted to the optical characterization of thin films, while SnO2/glass substrates present good electrical conductivity and are used in techniques where charge flows are needed. SnO2/glass substrates are also used in the final solar cell fabrication process.
Glass substrates are initially soaked for 10 min in aqueous hydrofluoric acid (HF) solution at 7%, then rinsed in distilled water. SnO2/glass substrates are prepared by spray pyrolysis of a 0.6 μm tin oxide thin film on glass [14,15].
SnxSy thin films are grown by CBD using two precursors, tin chloride SnCl2 with concentration equal to 2 × 10−2 M and Thioacetamide with concentration varying from 10−2 M to 8 × 10−2 M. Growth is carried out in an acid solution using acetic acid at pH = 0.41 [14], in order to prevent Sn(OH)2 precipitation. Infact, it precipitates from pH = 1.87 and tin concentration of 10−2 M according the solubility product KS1 = 5.45.1027 of following reaction: Sn2+ + 2OH− → Sn(OH)2 solid; whereas thioacetamide hydrolyses, when heated in strong acid solution according to the reaction:
| CH3CSNH2 + H2O + H3O+ → CH3COOH + NH4+ + H2S |
| Sn2+ + H2S → SnS + 2H+ |
Such experimental conditions are fulfilled as we chose to maintain the bath temperature at 80 °C.
3 Results and discussion
Complementary characterization techniques are applied to the obtained films in order to study different aspects related to their fabrication: crystallography, surface morphology, volume and surface composition. Glass or SnO2/glass substrates are used, and post-growth annealing is applied in some cases as mentioned below.
Tin sulphide thin film crystallography is studied using Philips XPer diffractometer and the (Cu)Kα energy (λ = 1.5418 Å). Thin films grown at 80 °C on glass and on SnO2/glass substrates present an amorphous structure as reported in the literature [2]. A crystal structure appears when the layers are annealed during 40 min at 320 °C under nitrogen gas. Fig. 1a and b represents XRD spectra of annealed layers on glass, for [Sn]/[TA] equal to 0.5 and 1 respectively. They exhibit two peaks at angular position 2θ = 14.84° for the first one, corresponding to Sn2S3 (d = 5.98 Å, ASTM 27-899), and 2θ = 31.82° for the second one, corresponding to Sn3S4 (d = 2.81 Å, ASTM 27-901). It can be noticed that there is no peak corresponding to SnS. Crystal growth mode is pointed out by the [Sn]/[TA] ratio: when [Sn]/[TA] is varied from 1 to 0.5, peak intensity of the Sn2S3 compound decreases while Sn3S4 peak increases slightly. Nevertheless, these two peaks exhibit low intensity and large full width at half maximum, which reveal the poor crystal structure of these films.

XRD spectra of tin sulphide layers grown by CBD: (a) on glass with [Sn]/[TA] = 1, (b) on glass with [Sn]/[TA] = 0.5, (c) on glass with [Sn]/[TA] = 0.25 and (d) on SnO2/glass with [Sn]/[TA] = 0.25.
Fig. 1c and d shows that thin films grown with [Sn]/[TA] = 0.25 exhibit a unique SnS crystal phase. The thin film grown on SnO2/glass substrate displays a better crystal structure than the one grown on glass. The full width at half maximum β is close to 0.625°; it corresponds to a polycrystalline layer whose crystallite mean size can be estimated to 13 nm, from application of the Scherrer law:
where K = 0.89 is the form factor, λ = 1.5418 Å, β = 0.625°, and θ the Bragg angle.
Surface topography is studied by AFM using a Veeco Metrology Group Dimension 3100 in contact mode. Fig. 2a, b and c corresponds to the surface of layers annealed after being deposited on glass with [Sn]/[TA] = 1, 0.5 and 0.25, respectively. Film surfaces present rough organisation. Surface RMS value decreases with the [Sn]/[TA] concentration ratio, from 43 nm at [Sn]/[TA] = 1, to 8 nm at [Sn]/[TA] = 0.5, and to 2.6 nm at [Sn]/[TA] = 0.25. Surface topography presented in Fig. 2d corresponds to the layer deposited on SnO2/glass substrate with [Sn]/[TA] = 0.25. It shows crystallites of different sizes placed side by side, with diameter varying from 10 nm to 100 nm.
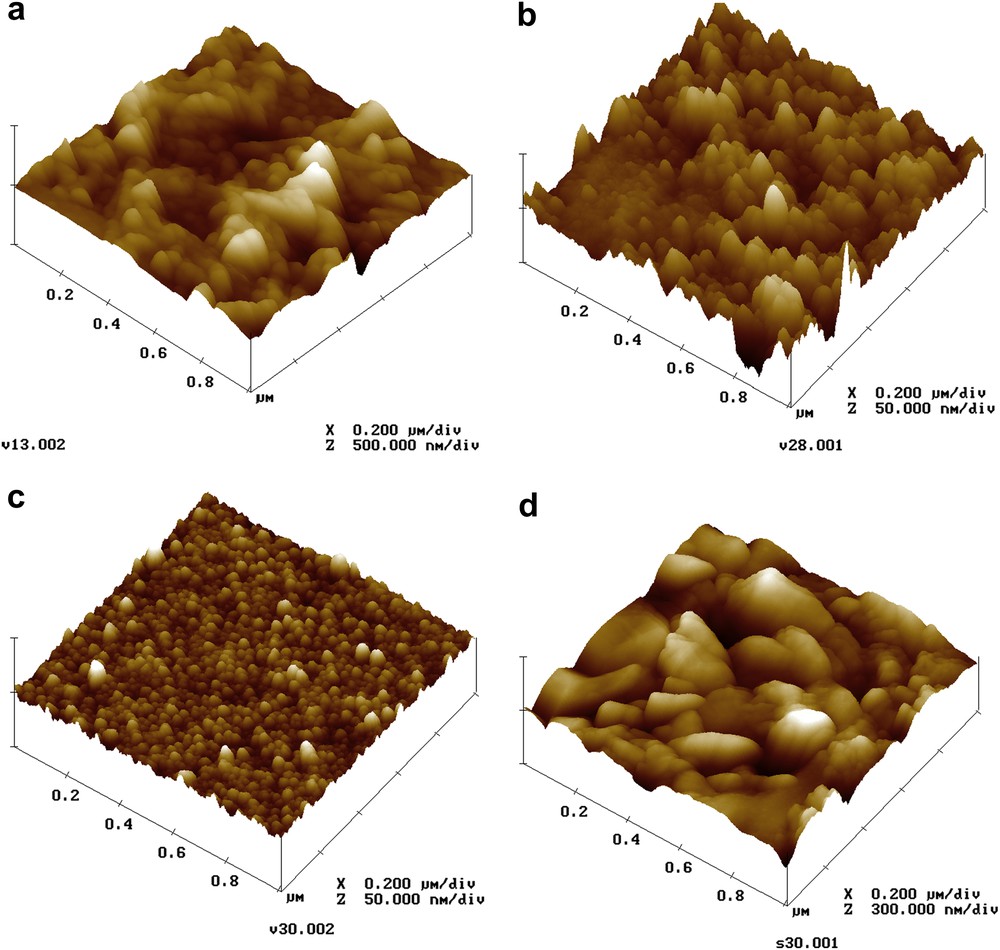
AFM of tin sulphide thin film surface obtained: (a) on glass with [Sn]/[TA] = 1, (b) on glass with [Sn]/[TA] = 0.5, (c) on glass with [Sn]/[TA] = 0.25 and (d) on SnO2/glass with [Sn]/[TA] = 0.25.
Chemical composition of the layers is studied by EDS using a LINK – AN10000 analyser. The films deposited on glass with [Sn]/[TA] varying from 0.25 to 2 are investigated. Results of this analysis are presented in Table 1. The Sn/S atomic ratio increases quasi-linearly with [Sn]/[TA]. The 1:1 stoichiometry is almost obtained at [Sn]/[TA] = 0.25. For [Sn]/[TA] above 0.25, chlorine is detected in the layer with weight percentage varying from 1.3 to 1.7%. It can be attributed to the precursor SnCl2 that is used for CBD. However, chlorine is not detected by EDS in the volume of the layer obtained with [Sn]/[TA] = 0.25.
EDS results of tin sulphide thin films deposited on glass by CBD with various values of [Sn]/[TA].
| [Sn] | [TA] | [Sn]/[TA] | (Sn/S)solid | (% Cl)solid |
| 2 × 10−2 M | 8 × 10−2 M | 0.25 | 1.09 | – |
| 2 × 10−2 M | 2.66 × 10−2 M | 0.75 | 1.16 | 1.77 |
| 2 × 10−2 M | 2 × 10−2 M | 1 | 1.38 | 1.45 |
| 2 × 10−2 M | 10−2 M | 2 | 1.75 | 1.35 |
Chemical analysis of the surface has been achieved using a RIBER CMA setup. The SnO2/glass substrate has been used as this analysis concerns the few topmost atomic surface layers. Fig. 3 represents AES spectrum of the layer grown with [Sn]/[TA] = 0.25. Tin and sulphur elements are identified, as well as carbon and oxygen pollution. Chlorine element is not present at the sample surface. The peak heights are then normalised using the sensitivity coefficient of the elements, and the atomic ratio Sn/S is found to be close to 1 at the surface.
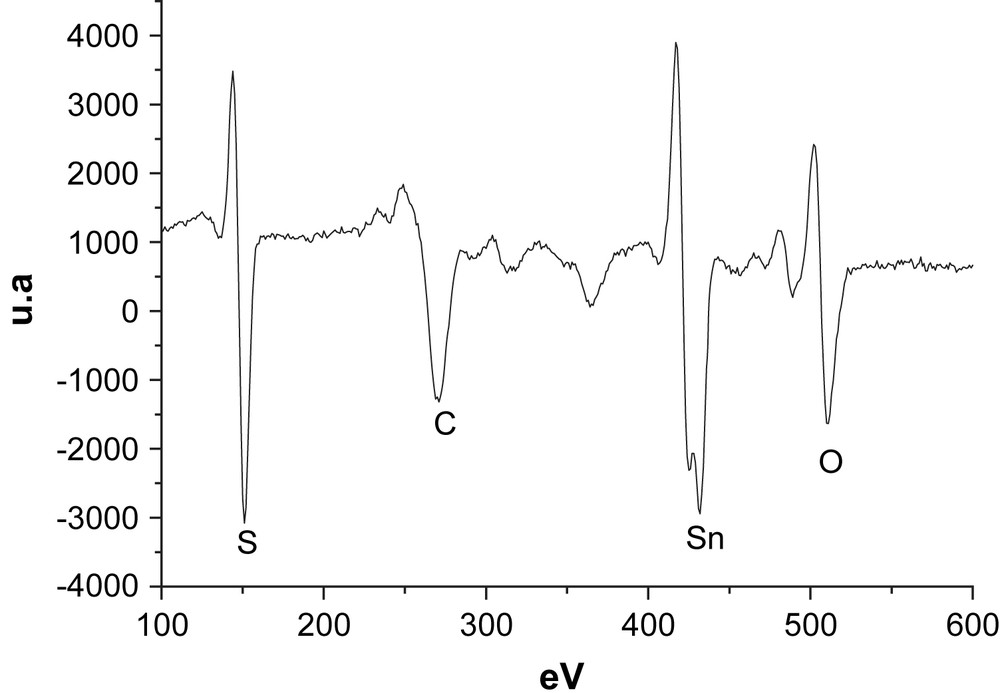
Auger spectra of tin sulphide thin film prepared on SnO2/glass by CBD with [Sn]/[TA] = 0.25.
4 Conclusion
Tin sulphide thin films can be grown by CBD in acid solution. The layers grown in an acid solution do not embed Sn(OH)2 solid phase that is usually observed in a basic solution. The layers grown on glass, then annealed during 40 min at 320 °C under nitrogen gas, exhibit poor crystal structure. This depends on the concentration ratio [Sn]/[TA] of the chemical bath taken between 0.25 and 1. Their surface do not reveal particular structure, from some 10 to some 100 nm. Layers obtained on SnO2/glass with [Sn]/[TA] = 0.25 show a dominating crystal structure corresponding to SnS compound in volume and at the surface. Crystal shapes of 100 nm diameter can be observed at the surface. Chlorine is not detected at the surface nor in the volume of the layers obtained with [Sn]/[TA] = 0.25. A few days after growth, impurities such as carbon and oxygen are detected at the surface of the layers, in noticeable amounts. It indicates that the layer surface is sensitive to ambient air conditions, and that it may be necessary to store tin sulphide thin films in good vacuum or inert gas environment, in the design of device processing.


