1 Introduction
Quinoxaline and its derivatives are an important class of benzoheterocycles displaying a broad spectrum of biological activities which have made them privileged structures in pharmacologically active compounds [1–4]. They have also been found applications as building blocks in the synthesis of organic semiconductors [5], rigid subunits in macrocyclic receptors or molecular recognition [6], and chemically controllable switches [7]. Recently, the synthesis of quinoxaline derivatives via the condensation of aryl 1, 2-diamines with 1, 2-dicarbonyl compounds in MeOH/AcOH [8] under microwave irradiation at 100 °C has been reported, but requires special instrumentation. In addition, improved methods have been developed for the synthesis of quinoxaline derivatives, including o-iodoxybenzoic acid [9], ceric(IV)ammonim nitrate [10], Yb(OTf)3 [11], H6P2W18O62·2H2O [12] and oxone [13]. However, most of the traditional processes suffer from a variety of disadvantages, such as pollution, high cost, poor chemical yields, requirements for long reaction time, and tedious work-up procedures, which limit their use under the aspect of environmentally benign processes. Recently, some other methods for the preparation of quinoxaline derivatives have been reported [14–16].
At the commencement of the new century, a shift in emphasis in chemistry is apparent with the desire to develop more environmentally friendly routes to a myriad of materials. This shift is most apparent in the growth of green chemistry [17–19]. Green chemistry approaches not only hold out significant potential for reduction of by-products, a reduction in the waste produced and lowering of energy costs, but also in the development of new methodologies towards previously unobtainable materials, using existing technologies [20].
Biopolymers, especially cellulose and its derivatives [21], have some unique properties, which make them attractive alternatives for conventional organic or inorganic supports for catalytic applications. Cellulose is the most abundant natural material in the world and it has been widely studied during the past decades because it is a biodegradable material and a renewable resource. Recently, science and technology are shifting emphasis on environmentally friendly and sustainable resources and processes. In this regard, biopolymers are attractive candidates to explore for supported catalysis [22,23]. Several interesting biopolymers have been utilized as a support for catalytic applications, such as alginate [24], gelatin [25,26], starch [27] and chistosan [28] derivatives.
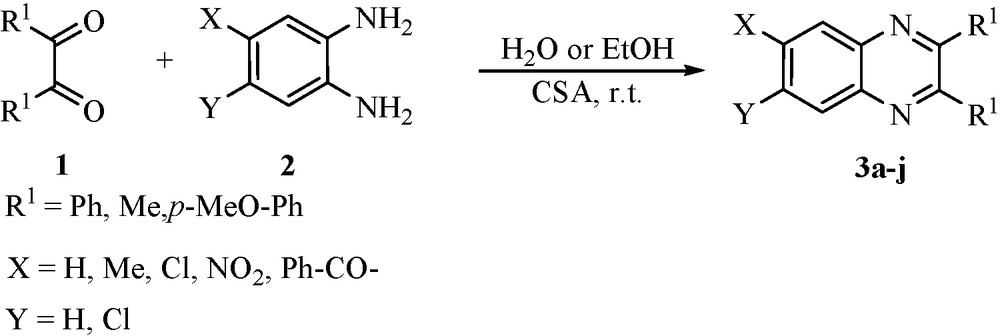
During the course of our studies toward the development of new routes to the synthesis of heterocyclic compounds using green reaction mediums [29–33], we herein disclose a valid and an efficient procedure for the synthesis of quinoxaline derivatives via condensation of 1,2-diketones: (1) with o-diamines; (2) in the presence of cellulose sulfuric acid (CSA) [34–36], as an inexpensive and biodegradable solid acid catalysts in H2O or EtOH at room temperature (Scheme 1).
2 Results and discussion
The synthesis of 3a–j was accomplished as outlined in Scheme 1. Reaction of the appropriate 1,2-diketone: (1) with o-diamino-substituted benzene; (2) in the presence of CSA gave the known quinoxaline derivatives in high yields at room temperature. The catalyst is very active, stable to air and moisture, nontoxic and inexpensive. In addition, it can be quantitatively recovered by filtration and reused. Both aliphatic and aromatic 1,2-diketones afforded good yields. The results summarized in Table 1 clearly indicate the scope and generality of the reaction with respect to various 1,2-diketones and o-diamines. As can be seen from Table 1, in comparison of EtOH and H2O as a solvent, EtOH is better solvent in view point of short reaction times and improvement of yields. Most of the reactions proceed very cleanly at room temperature and no undesirable side-reactions were observed, although the yields were highly dependent on the substrate used (spatially in H2O). For instance, a substrate bearing a strong electron withdrawing group completely stopped in H2O even after 24 h (Table 1, entries 3c and 3h).
Synthesis of quinoxaline derivatives using CSA as catalyst in EtOH and H2O.
| Entry | R1 | X | Y | EtOH (time / yield (%)a) | H2O (time / yield (%)a) |
| 3a | Phenyl | H | H | 60 min / 93 (92, 95, 90, 90)b | 2.30 h / 80 (78, 80, 82, 80)b |
| 3b | Phenyl | Me | H | 75 min / 90 | 2.30 h / 72 |
| 3c | Phenyl | NO2 | H | 6 h / 86 | 24 h / 0 |
| 3d | Phenyl | Cl | Cl | 100 min / 90 | 24 h / 80 |
| 3e | Phenyl | Ph-CO- | H | 3 h / 92 | 24 h / 83 |
| 3f | Me | Me | H | 2 h / 86 | 2 h / 75 |
| 3g | Me | Cl | Cl | 2 h / 91 | 2 h / 77 |
| 3h | Me | NO2 | H | 2 h / 79 | 2 h / 0 |
| 3i | 4-Methox phenyl | H | H | 24 h / 85 | 24 h / 50 |
| 3j | 4-Methox phenyl | Me | H | 24 h / 81 | 24 h / 50 |
a Isolated yields.
b The yields of four subsequent runs by using the same recovered catalyst.
Cellulose sulfuric acid is readily prepared [34–36] by the dropwise addition of chlorosulfonic acid to a CHCl3 mixture of cellulose at 0 °C. It is important to note that this reaction is easy and clean without any work-up procedure since the HCl gas is evolved from the reaction vessel immediately. This white homogeneous, non-hygroscopic solid acid is very stable under reaction conditions (Scheme 2).
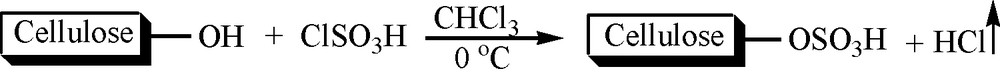
To illustrate the need for cellulose sulfuric acid for these reactions, the experiment (Table 1, entry 3a) was conducted in the absence of cellulose sulfuric acid. The yield in the absence of catalyst was about 20% after 60 min. Obviously, the cellulose sulfuric acid is an important component of the reaction.
In order to obtain the best solvent, benzil: (1) with benzene-1,2-diamine; (2) in the presence of CSA in water, various organic solvents and ionic liquid were allowed to react at room temperature. As can be seen from Table 2, EtOH is the best solvent respect to yield and short reaction time (Table 2, entry 2). In the case of H2O the reaction time is long for the reasonable yield (Table 2, entry 1).
Effect of solvent on the reaction times and yields.
| Entry | Solvent | Time | Yield (%) |
| 1 | H2O | 2.30 h | 72 |
| 2 | EtOH | 60 min | 93 |
| 3 | CH3CN | 3 h | 60 |
| 4 | C6H5CH3 | 3 h | 75 |
| 5 | n-Hexane | 3 h | 77 |
| 6 | [bmim]Br | 3 h | 85 |
One of the advantages of solid acid catalysts is their ability to perform as a recyclable reaction media. We were able to separate CSA from the reaction medium easily by washing with CH2Cl2. After drying it was reused for subsequent reactions (Table 1, entry 1). Thus, this process could be also interesting for large-scale synthesis.
3 Experimental
3.1 Materials and techniques
Melting points were taken on an Electrothermal 9100 apparatus and left uncorrected. IR spectra were obtained on a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were recorded on a Bruker DRX-300 Avance spectrometer at 300.13 and 75.47 MHz. NMR spectra were obtained on solutions in CDCl3 or DMSO using TMS as internal standard. All of the chemicals were purchased from Fluka, Merck and Aldrich and used without purification. All reaction products were known and characterized by IR, 1H and 13C NMR spectra and melting point as comparing with those obtained from authentic samples.
3.2 General procedure for preparation of quinoxaline derivatives
In a typical reaction, a mixture of 1,2-diketone (1 mmol) and o-diamine (1 mmol) in EtOH or H2O in the presence of cellulose sulfuric acid (0.01 g) was stirred for suitable time at room temperature. Reaction progress was monitored by TLC. After completion of the reaction, the reaction mixture was filtered and washed with CH2Cl2 (10 mL) to separate the catalyst. Then the filtrate's solvent was evaporated under reduced pressure and chromatographed over silica gel (ethyl acetate/n-hexane, 1/5) to afford a pure product.
4 Conclusions
In conclusion, cellulose sulfuric acid as an efficient and environmentally friendly bio-supported proton source catalyst was prepared and employed for the synthesis of quinoxaline derivatives via the condensation of 1,2-diketone with o-diamine in H2O or EtOH as a green solvent in relatively high yields at room temperature. Good yields, recyclability of the catalyst with no loss in its activity, easy work-up procedures, the use of green solvent, the use of nontoxic, noncorrosive and an inexpensive solid acid catalyst are important features of this new protocol to prepare quinoxaline derivatives.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.


