1 Introduction
Noble metals, including palladium(II) (Pd[II]), are metals of lower natural abundance having increasing applications in electric and electronic components and as high-performance catalysts. Keeping in mind these considerations, the recovery of these metals is almost a required task to meet future demands. Solvent extraction is one of the well-known methods used for noble metals-chloride recovery and separation. Several extractants have been suggested, including hydroxyoximes [1], hydrophobic amines [2], dialkyl sulphides [3], organophosphoric acids [4] and azamacrocyclic ligands [5].
Nowadays, the development of more selective and tailored extractants is observed. For example, calixarenes, cyclic oligomers of phenol-formaldehyde condensates have received the particular interest of numerous chemists in recent years, because of their ease of synthesis and their ionic and molecular-binding properties [6]. The chemical modification of the upper or the lower rim of these calixarenes provides a great variety of macrocycles having different complexation ability and different conformational behaviour. Recently, we have investigated the extraction and separation of some noble metals ions from chloride solutions using thiacalix[4]arene derivatives [7–9].
In the present work, an azocalix[4]arene derivative (ligand 1) has been tested as extractant for noble metals from acidic chloride media. Only Pd(II) was quantitatively extracted using the tested ligand. A kinetic study to determine the equilibrium time and the effect of the chloride concentration on the Pd(II) extraction efficiency was performed. The molar ratio method was used to determine the complex stoechiometry in acetonitrile as solvent.
2 Experimental
2.1 Reagents and solutions
Stock solution of palladium (500 mg L–1 in 1 M HCl) was prepared by dissolving a weighted amount of Pd(II) chloride (Merck, Germany) in concentrated HCl. Working solutions containing different amounts of the metal were prepared by dilution of the stock solution with deionized water (MilliQ Plus column, Millipore), and resulting pH was 1.7. A standardized NaCl solution was used to adjust chloride amount of aqueous Pd(II) solutions.
The azocalix[4]arene derivative tested in this work, 5,17-bis(phenylazo)-26,28-dihydroxy-25,27-di(ethoxycarbonylmethoxy)calix[4]arene (ligand 1; Fig. 1), was synthesized using the procedure described by Halouani et al. [10].

Chemical structure of ligand 1.
2.2 Liquid-liquid extraction procedure
2 mL of acidic aqueous solution containing 10-4 M of palladium and 2 mL of a 4 × 10-3 M ligand 1 solution in chloroform were mixed in stoppered glass tubes and shaken at 40 rpm and at 22 ± 1 °C. The mixture was then centrifuged and the two phases were separated. The concentration of metallic ions in aqueous solutions was determined by atomic emission spectrometer (ICP-AES) type Varian Liberty RL. The percentage of extraction (%E) was calculated from the concentration of metal ions before ([M]aq,init) and after ([M]aq) extraction:
| (1) |
The recovery of the extracted metal in the separated organic phase (2 mL) was investigated by using 2 mL of some tested stripping solutions. The percentage of recovery (%R) was calculated from the concentration of stripped metal ions ([M]aq,stri) and the concentration of extracted metal ions in the organic phase ([M]aq,init - [M]aq):
| (2) |
The distribution ratio (D) was calculated from the aqueous Pd(II) concentration before and after equilibrium:
| (3) |
2.3 Complexation study
The recognition properties between Pd(II) and ligand 1 in acetonitrile solution were investigated by UV-visible titrations (λ ranging from 200 to 650 nm). Absorption measurements were carried out at 20 °C and were recorded on a Shimadzu UV-2401 PC spectrophotometer.
3 Results
3.1 Liquid-liquid extraction experiments
3.1.1 Extraction and stripping efficiencies
The recognition properties of ligand 1 towards noble metals has been evaluated using 10-4 M metal solutions in 0.02 M HCl (pH = 1.7) in the case of Au(III), Pt(IV), Pd(II) and Rh(III), whereas silver solution was buffered at pH 6 using 2-(N-morpholino)-ethane sulphonic acid (MES) buffer. The extraction efficiencies obtained after 24 hours of phases contact are presented in Table 1.
Extraction efficiencies of ligand 1 towards noble metals.
| Metal | E (%) |
| Ag(I) | 29.5 |
| Au(III) | 8.6 |
| Pd(II) | 96.8 |
| Pt(IV) | 0 |
| Rh(III) | 0 |
It can be seen that the extraction efficiency of gold, silver, platinum or rhodium is lower than that observed for palladium. This efficiency is even zero in the case of Pt(IV) and Rh(III). In contrast, Pd(II) is almost completely extracted under the experimental conditions mentioned above, and hence, further liquid-liquid extraction experiments were performed for this metal.
The stripping efficiency of Pd(II) was tested using the separated organic solution contacted during 24 hours with 2 mL of some stripping solutions listed in Table 2 with their recovery efficiencies. From this table, it can be observed that thiocyanate, thiourea or thiourea in 1 M HCl solutions can achieve more than 50% back-extraction of Pd(II), but a complete stripping was reached only using 0.5 M thiourea in 1 M HCl.
Recovery efficiencies for Pd(II) extracted with ligand 1 using different stripping solutions.
| Stripping solution | R (%) |
| 0.5 M thiourea | 67.8 |
| 0.5 M thiourea in 1 M HCl | 100 |
| 1 M HCl | 14.1 |
| 0.5 M NaSCN, pH 2 | 92.0 |
| 0.1 M Na2S2O3 | 26.3 |
3.1.2 Study of the kinetics of extraction of Pd(II)
Kinetics of Pd(II) extraction has been studied using 10-4 M Pd(II) solution in 0.02 M HCl (pH = 1.7) mixed with 4 × 10-3 M of ligand 1 solution in chloroform at different contact times. The variation of %E as a function of time is given in Fig. 2. From this variation, it was observed that the percentage of extraction was more or less constant after 500 minutes of phase contact. The maximum extraction of Pd(II) reached about 95% when the phases were equilibrated at least for 25 hours. The prolongation of phase contact time to over 25 hours had only a slight effect on the yield of Pd(II) extraction. Nevertheless, 24 hours of the phase contact time was selected as sufficient to reach apparent equilibrium and/or optimal extraction percentage. This equilibrium time can be considered as long compared to the 60 minutes obtained by Rane and Venugopal [11] when using 2-hydroxy-5-nonylacetophenone oxime (LIX84 I) as palladium extractant but calixarene 1 showed better selectivity. In fact, LIX84 I can also extract poorly Pt(IV) from hydrochloric solutions and quantitatively from ammoniacal solutions, whereas the used azocalixarene extract quantitatively only Pd(II) among all the tested noble metals.
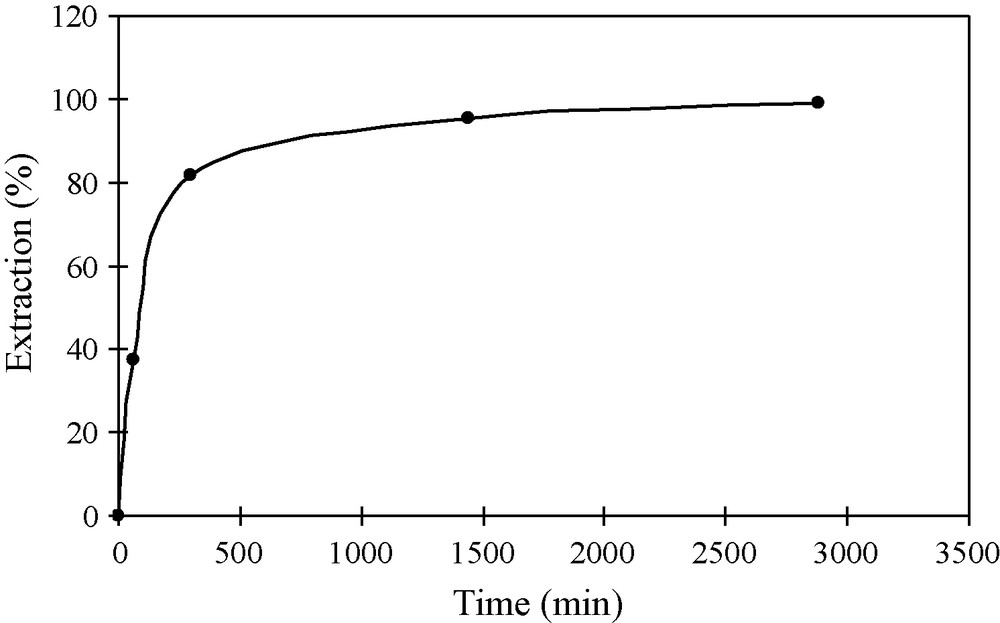
Determination of equilibrium time for extraction of Pd(II) from acidic chloride media with ligand 1. Aqueous phase: 2 mL of 10-4 M Pd(II) ions in 0.02 M HCl (pH = 1.7). Organic phase: 2 mL of a chloroform solution of 4 × 10-3 M ligand 1. Temperature: 22 ± 1̊C.
3.1.3 Effect of chloride concentration in the aqueous phase
The percentage of extraction of Pd(II) with ligand 1 was determined at the same experimental conditions mentioned above and at different chloride concentrations. This concentration was varied from 0.02 to 2 M using standardized NaCl solutions. Obtained results indicate that palladium extraction percentage decreases with increasing chloride concentration. The presence of high chloride ions amounts strongly diminishes the extraction efficiency and no significant extraction was revealed at 0.65 M chloride concentration. Therefore, it can be concluded that the equilibrium established between Pd(II) and ligand 1 depends on the chloride concentration of the aqueous phase. The log-log plot of distribution ratios, D, against the chloride concentration in the aqueous phase permits an estimation of the number of chloride ions involved in the extraction scheme [12,13]. In fact, the plot of the distribution ratios (log D) versus the chloride concentration (log [Cl-]) was found to be a straight line with negative slope of approximately four as shown in Fig. 3. Taking into account that the predominant species under these chloride concentrations was PdCl42-, the extraction of Pd(II) ions from acidic chloride media with ligand 1 (L) can be described as follows:
| (4) |
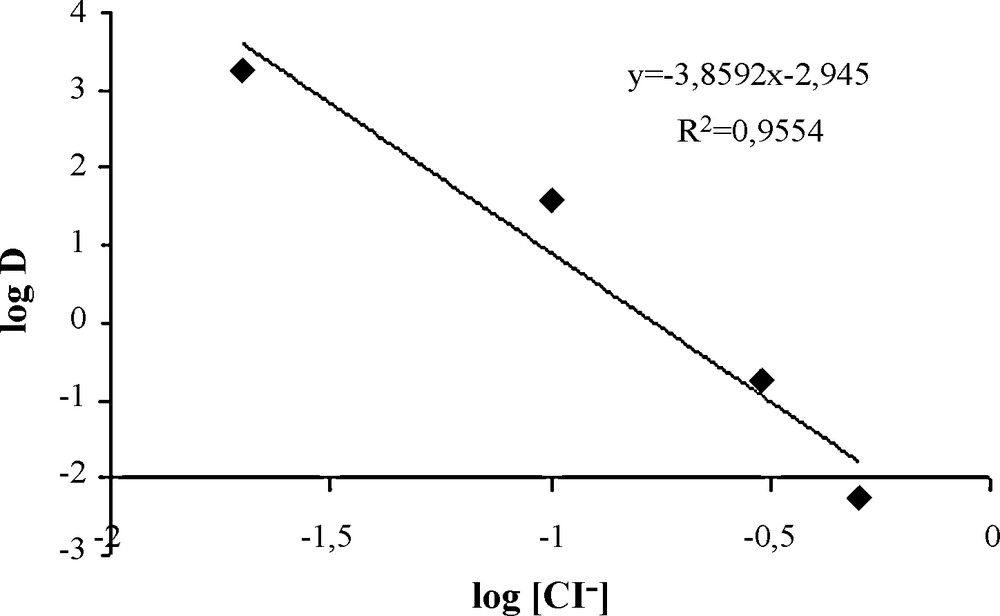
Plot of log D versus log [Cl-]. Aqueous phase: 2 mL of 10-4 M Pd(II) ions in 0.02 M HCl (pH = 1.7) and different chloride concentrations (standardized NaCl solutions). Organic phase: 2 mL of a chloroform solution of 4 × 10-3 M ligand 1. Temperature: 22 ± 1̊C. Contact time: 24 hours (40 rpm).
Based on the proposed extraction mechanism, it is clear that the increase in the chloride concentration favours the formation of PdCl42- species and as a result, a decrease in the extraction efficiency is expected [12].
3.2 Complexation study
To confirm the above Pd(II) extraction scheme, a complexation study has been performed. In fact, the binding ability of compound 1 towards Pd(II) ions was investigated by UV-Vis absorption method. Increasing amounts of solution of metal ion (10-3 mol.L-1) were added with a microliter syringe (Hamilton, 10 μL) into a cell containing a known volume of the ligand solution (1 mL, 5 × 10-5 mol.L-1 in acetonitrile). The solution was allowed to equilibrate for 5 minutes. Titration curves of the ligand 1 upon addition of Pd(II) in a neutral medium are shown in Fig. 4. A large decrease of the absorbance around 356 nm is observed and at this wavelength, the molar ratio study shows the absence of any interaction between ligand 1 and Pd(II). Nevertheless, a slight isosbestic point appears at 400 nm; also a very weak complex may exist in the neutral medium. These results were confirmed by the liquid-liquid extraction experiments where no extraction of Pd(II) ions by the macrocycle 1 in neutral medium was mentioned. Furthermore, the complexation study was also realized in acidic medium. Increasing amounts of solution of metal ion (10-3 mol.L-1) acidified with 0.02 mol of HCl (37%) were added with a microliter syringe (Hamilton, 10 μL) into a cell containing a known volume of the ligand solution (1 mL, 5 × 10-5 mol.L-1 in acetonitrile). The solution was allowed to equilibrate for 5 minutes. Upon addition of Pd(II) solutions, the UV–Vis ligand spectrum undergoes clean changes that indicate the formation of at least one metal complex species. The addition of aliquots of Pd(II) (from 0.2 to 3 equiv.) to a solution of ligand 1, leads to the appearance of a new MLCT absorption band (Metal Ligand Charge Transfer band) centred at 486 nm (Fig. 5). Moreover, the presence of isosbestic points at 323 and 397 nm indicates the existence of a new species.
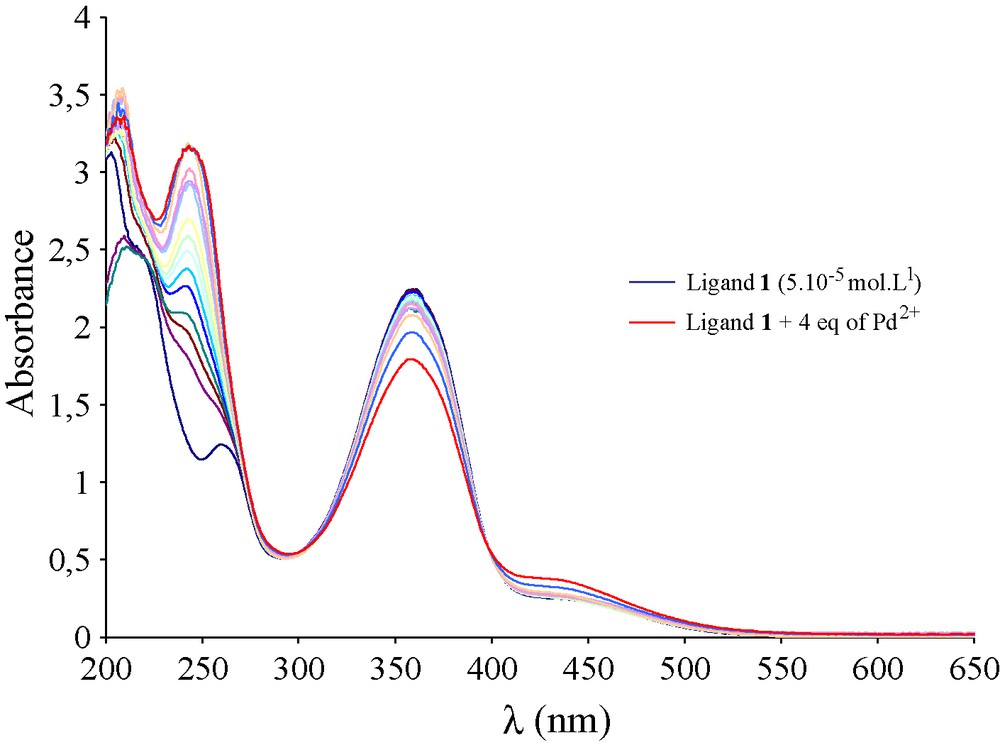
UV-Vis titration curves of the ligand 1 (5 × 10-5 mol L-1 in acetonitrile) upon addition of four equivalents of Pd(II) in neutral medium (10-3 mol L-1).
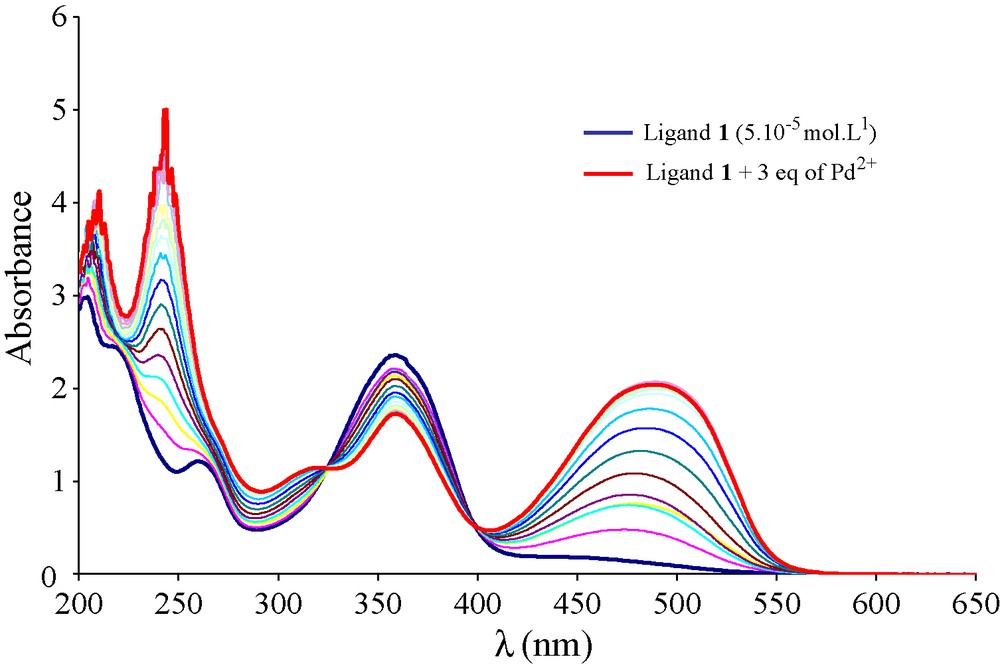
UV-Vis titration curves of the ligand 1 (5 × 10-5 mol L-1 in acetonitrile) upon addition of three equivalents of Pd2+ in acidic medium (10-3 mol L-1 + 0.2 mol HCl).
The stoechiometry of the complex formed between the metal chloride species and the ligand was determined by the molar ratio method [14]. The ratio molar graph was obtained from UV measurements at 486 nm (Fig. 6) and showed that a 2:1 Pd(II)-ligand 1 complex was formed. This result confirmed the stoechiometry given in the above proposed extraction scheme (4).
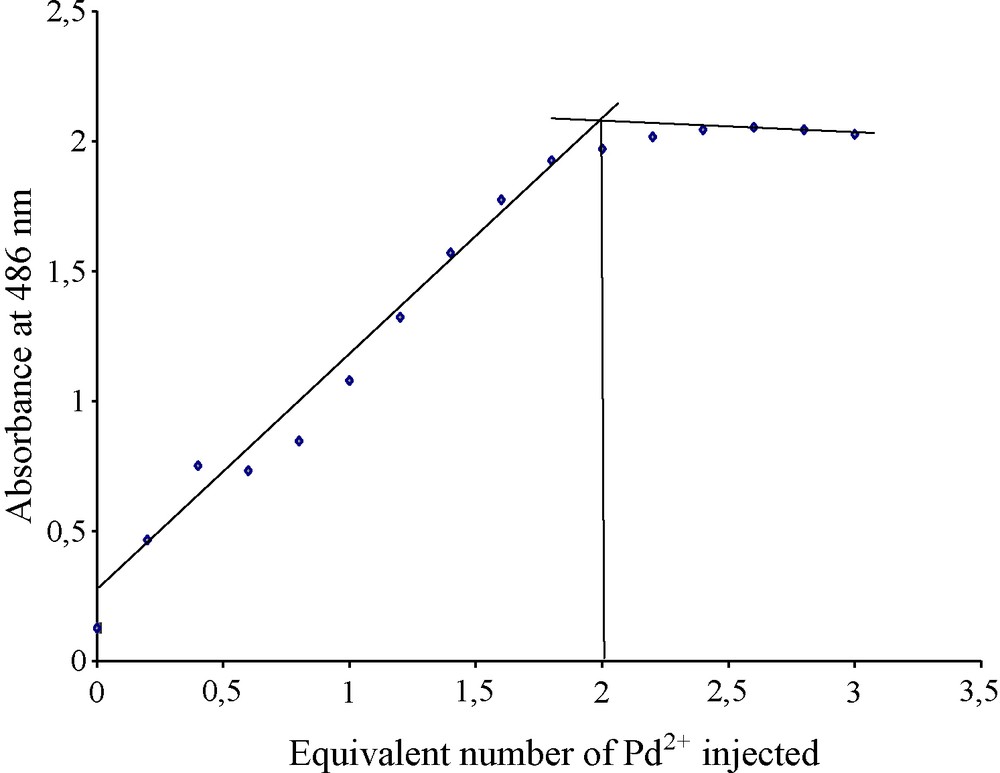
Ratio molar graph from the UV-Vis titration curves of the ligand 1 (5 × 10-5 mol L-1 in acetonitrile) upon addition of three equivalents of Pd2+ in acidic medium at 486 nm.
The results obtained by liquid-liquid extraction and by the complexation study are complementary and showed that interaction between Pd(II) ions and calixarene 1 was only possible in hydrochloric acid solutions.
Acknowledgements
This study has been financed by CYCIT project CTQ2005-09430-C05-01. The authors are grateful to the Spanish-Tunisian cooperation project A/4027/05 and the French “Rhone-Alpes” region for the MIRA project. The authors would like to thank Dr. C. Fontàs and Dr I. Bonnamour for experiments and fruitful discussions.


