1 Introduction
The importance of fused pyrimidines, common source for the development of new potential therapeutic agents [1,2], is well known. Among them, the pyridopyrimidine scaffold and their oxo and thioxo derivatives is a well-known pharmacophore in drug design and it is associated with a wide range of biological properties [3–7]. Pyrido[3,2-d]-pyrimidines have been described as tyrosine kinase inhibitors and as dihydrofolate reductase inhibitors [8,9]. Pyrido[2,3-d]pyrimidines possess dihydrofolate reductase inhibiting and antitumour activity [10]. Similarly, in recent years, considerable attention has been focused on the development of new methodology to synthesize many kinds of pyrazolopyrimidine ring system [11]. Indeed, these compounds are, by now widely recognized as important organic materials showing interesting biological activities [12]. Considering the important biological properties of fused pyrimidines, a number of methods have been reported for the synthesis of these heterocycles [13–20].
One of the powerful tools used to combine economic aspects with the environmental concerns is performing organic reactions in water; this strategy consists of two or more synthetic steps, which are carried out in water as a cheap, nontoxic, environmentally friendly solvent, in a one-step reaction, without isolation of any intermediate thus reducing time, saving money, energy and raw materials [21,22]. The development and implementation of processes using water as solvent may serve as one avenue for lowering both the environmental impact of the chemical industry and the hundred billion dollar costs associated with environmental regulation compliance [23]. Moreover, multicomponent condensation reactions due to their productivity, simple procedures, convergence and facile execution are one of the important strategies in combinatorial chemistry [24,25]. As a result, one-pot multicomponent reactions in organic chemistry have expanded rapidly [26,27].
Due to the biological activity of fused pyrimidines, and our interest in the synthesis of heterocyclic compounds especially pyrimidine derivatives [28–42], herein, we report a clean and efficient method for the preparation of pyrimidine-fused heterocycles in water.
2 Results and discussion
It was found that a mixture of 1,3-dimethyl-6-amino-uracil 1a, benzaldehyde 2a and barbituric acid 3a in the presence of catalytic p-toluene sulfonic acid (p-TSA) as an inexpensive and readily available catalyst afforded 1,3-dimethyl-5-phenyl-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone 4a in 86% yield in refluxing water for 4 h (Scheme 1).
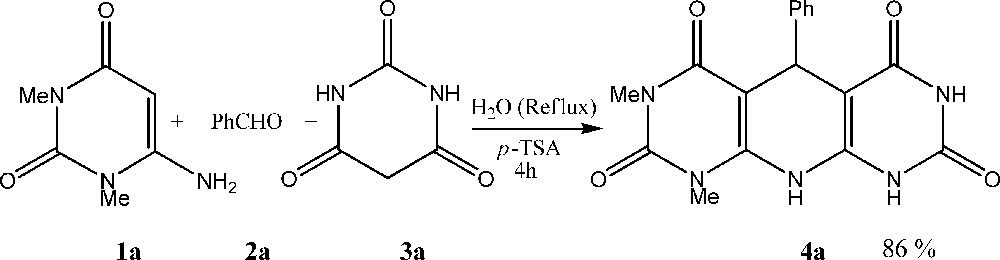
Then, to delineate this approach, particularly in regard to library construction, this methodology was evaluated by using different barbituric acids, aldehydes and 6-amino-uracils. Three uracils 1a-c, four substituted aromatic aldehyds 2a-d and three barbituric acids 3a-c were chosen for the library validation (Scheme 2). Corresponding pyrido[2,3-d:6,5-d]dipyrimidine derivatives 4a-l were synthesized by the one-pot, three-component condensation in good yields at refluxing water in the presence of p-TSA for 4 h. The reaction can be represented as in Table 1.
Synthesis of pyrido[2,3-d:6,5-d]dipyrimidines 4a-l.
| Entry | Compound 1 | Ar | Compound 3 | Product 4 | Yield (%)a |
| 1 | C6H5 2a | 86 | |||
| 2 | 1a | 4-ClC6H4 2b | 3a | 87 | |
| 3 | 1a | 4-NO2C6H4 2c | 3a | 89 | |
| 4 | 1a | 4-MeOC6H4 2d | 3a | 86 | |
| 5 | 1a | C6H5 2a | 82 | ||
| 6 | 1a | 4-ClC6H4 2b | 3b | 81 | |
| 7 | 1a | 4-NO2C6H4 2c | 3b | 84 | |
| 8 | C6H5 2a | 3b | 4a | 81 | |
| 9 | 1b | -ClC6H4 2b | 3b | 4b | 83 |
| 10 | 1b | 4-NO2C6H4 2c | 3b | 4c | 85 |
| 11 | 1b | 4-MeOC6H4 2d | 3b | 4d | 77 |
| 12 | 1b | C6H5 2a | 80 | ||
| 13 | 1b | 4-ClC6H4 2b | 3c | 81 | |
| 14 | C6H5 2a | 3a | 4h | 84 | |
| 15 | 1c | 4-ClC6H4 2b | 3b | 4i | 86 |
| 16 | 1c | C6H5 2a | 3b | 81 | |
| 17 | 1c | 4-ClC6H4 2b | 3b | 88 | |
| 18 | 1a | C6H5 2a | 3c | 4j | 85 |
| 19 | 1a | 4-ClC6H4 2b | 3c | 4k | 87 |
a Isolated yields.
The results were good in terms of yields and product purity in the presence of p-TSA, while without p-TSA and over long period of time (12 h) the yields of products were low (<40%). When this reaction was carried out with an aliphatic aldehyde such as propionaldehyde or butanaldehyde in same conditions (water/p-TSA), TLC and 1H NMR spectra of the reaction mixture showed a combination of starting materials and numerous products, the yield of the expected product was very poor.
The nature of these compounds as 1:1:1 adducts was apparent from their mass spectra, which displayed, in each case, the molecular ion peak at appropriate m/z values. Compounds 4 are stable solids whose structures are fully supported by IR, 1H and 13C NMR spectroscopy, mass spectrometry, and elemental analysis.
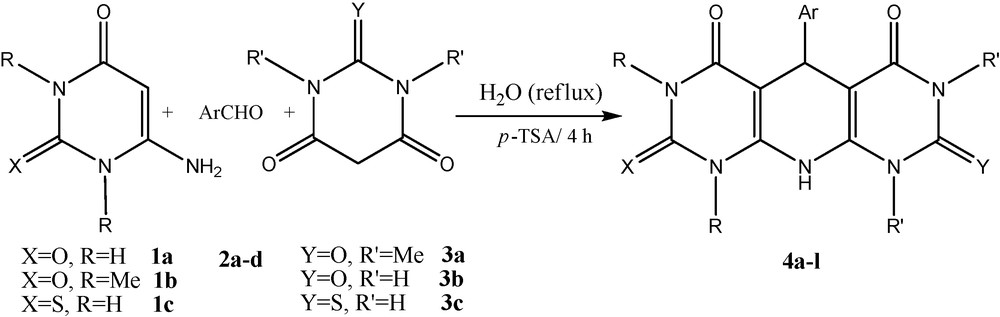
A possible mechanism for the formation of 4 is proposed in Scheme 3. It is reasonable to assume that 4 results from initial formation of a heterodiene 5 by standard Knoevenagel condensation of the barbituric acid 3 and aldehyde 2. Then, the subsequent Michael-type addition of the 6-amino-uracil 1 to the heterodyne 5, followed by cyclization affords the corresponding products 4 (Scheme 3).
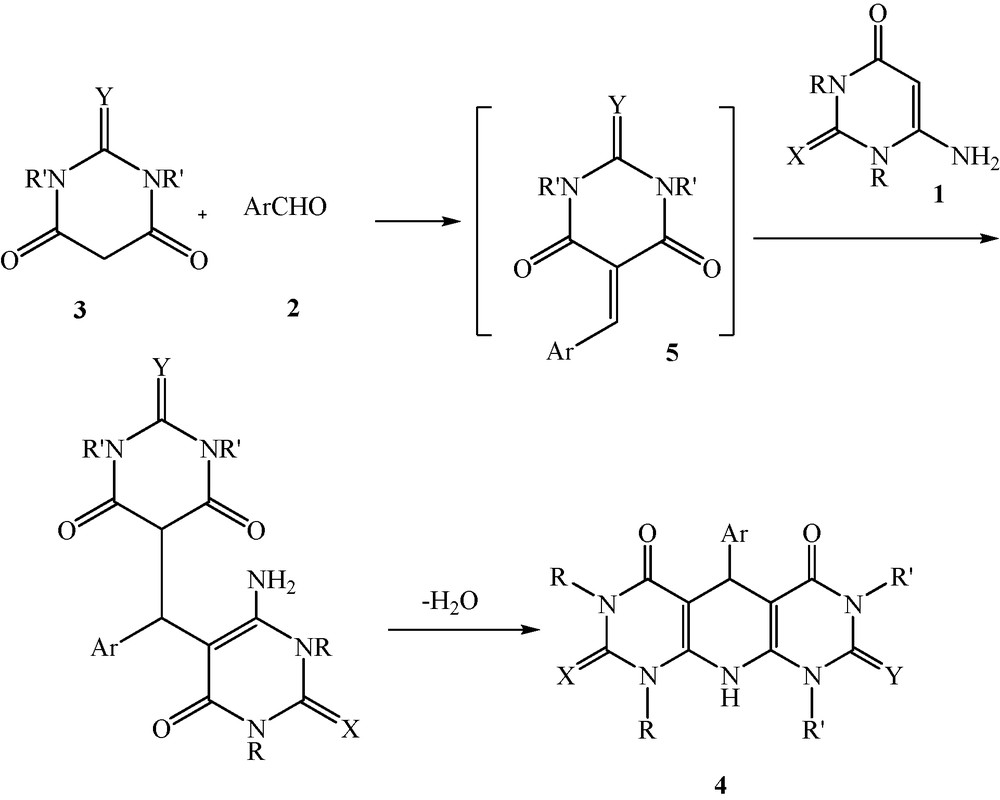
Very recently, we reported the synthesis of pyrazolo[4’,3’:5,6]pyrido[2,3-d]pyrimidine-diones under solvent-free conditions [43]. Finally, to further explore the potential of this clean protocol for pyridopyrimidines synthesis, we investigated reaction involving 1H-pyrazol-5-amines 6, barbituric acids 3 and aromatic aldehyde 2 and obtained pyrazolo[4’,3’:5,6]pyrido[2,3-d]pyrimidine-dione derivatives 7a-l in good yields for 3 h (Scheme 4, Table 2).
Synthesis of 1H-pyrazolo[4’,3’:5,6]pyrido[2,3-d]pyrimidinones 7.
| Product 7 | Ar | X | Y | Yielda (%) |
| a | C6H5 | H | O | 81 |
| b | 4-ClC6H4 | H | O | 82 |
| c | 4-BrC6H4 | H | O | 80 |
| d | C6H5 | NO2 | O | 79 |
| e | 4-ClC6H4 | NO2 | O | 86 |
| f | 4-BrC6H4 | NO2 | O | 81 |
| g | C6H5 | H | S | 89 |
| h | 4-ClC6H4 | H | S | 83 |
| i | 4-NO2C6H4 | H | S | 80 |
| j | C6H5 | NO2 | S | 85 |
| k | 4-ClC6H4 | NO2 | S | 83 |
| l | 4-NO2C6H4 | NO2 | S | 84 |
a Isolated yields.
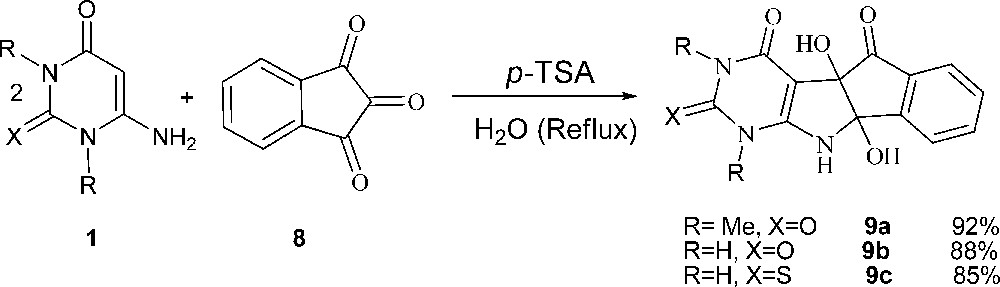
To further explore the potential of this protocol for pyrimidine-fused heterocyles synthesis, we investigated reaction of 6-amino-uracils 1 with ninhydrine 8 and obtained tetrahydroindeno[2’,1’:4,5]pyrorolo[2,3-d]pyrimidine-trione derivatives 9a-c in good yields (Scheme 5).
Finally, compounds 4 and 9 were screened for antimicrobial activity. The microorganisms used in this study were Escherichia coli ATCC 25922, Pseudomonas aeruginusa ATCC 85327, (Gram-negative bacteria), Enterococcus faecalis ATCC 29737, Bacillus subtilis ATCC 465, Bacillus pumilus PTCC 1114, Micrococcus luteus PTCC 1110, Staphylococcus aureus ATCC 25923, Staphylococcus epidermidis ATCC 12228, Sterptococcus mutans PTCC 1601 (Gram-positive bacteria). The minimum inhibitory concentration (MIC) of the synthesized compounds determined by microdilution method [44] and compared to two commercial antibiotics (Tables 3 and 4).
MIC (μg/ml) values of products 4.
| Products | Standard | |||||||||||||
| 4a | 4b | 4c | 4d | 4e | 4f | 4g | 4h | 4i | 4j | 4k | 4l | Tetracycline | Gentamicin | |
| Bacillus subtilis | 64 | 32 | 32 | 64 | 32 | 8 | 32 | 16 | 8 | 16 | 16 | 16 | 4 | a |
| Bacillus pumilus | 18 | 14 | a | 16 | a | 16 | a | 32 | 16 | 16 | 16 | 32 | 8 | a |
| Micrococcus luteus | 32 | 32 | a | 128 | a | 32 | a | 16 | 8 | 16 | 16 | a | 4 | a |
| Staphylococcus aureus | a | 128 | a | a | a | 16 | a | 32 | 16 | 16 | 8 | 32 | 4 | a |
| Staphylococcus epidermidis | 64 | 32 | 64 | 16 | a | 8 | 256 | 32 | 16 | 16 | 16 | 16 | < 2 | a |
| Sterptococcus mutans | 64 | 32 | 64 | 64 | 256 | 8 | 32 | 16 | 4 | 8 | 4 | 32 | 2 | a |
| Escherichia coli | 16 | 4 | 8 | 4 | 4 | 8 | 16 | 32 | 16 | 16 | 16 | 8 | a | 4 |
| Enterococcus faecalis | 2 | < 2 | < 2 | < 2 | < 2 | < 2 | 4 | 6 | 4 | 4 | 4 | 2 | 8 | a |
| Pseudomonas aeruginosa | 12 | 8 | 8 | 4 | 8 | 4 | 8 | 2 | < 2 | 2 | < 2 | 2 | a | 8 |
MIC (μg/ml) values of products 9.
| Compounds | Microorganisms | ||||||||
| B. subtilis | B. pumilus | M. luteus | S. aureus | S. epidermidis | S. mutans | E. coli | E. faecalis | P. aeruginosa | |
| 9a | 8 | 4 | 14 | 24 | 12 | 18 | 2 | 2 | 4 |
| 9b | 12 | 8 | 24 | 32 | 16 | 24 | 4 | 2 | 6 |
| 9c | 6 | 4 | 12 | 16 | 8 | 14 | 2 | 2 | 4 |
Most of the compounds have a narrow to good spectrum antimicrobial activity. All of the compounds were found to be more active than Tetracycline against E. Faecalis (<2–-6 μg/ml), E. coli (4–32 μg/ml) and P. aeruginosa (<2–16 μg/ml). Almost all of the compounds were found to be more active than Gentamicin against all tested strains. The products 4a, 4c-e and 4 g do not have antibiotic activity against S. aureus. Although, all of the compounds were found to be less active than Tetracycline against the B. subtilis, B. pumilus, M. luteus, S. aureus, S. epidermidis, S. mutans. 5-Aryl-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H,5H,7H)-triones 4h-l were more active than the corresponding ones of the series 4a-g, reinforcing the pharmacophoric contribution of thiocarbonyl moiety relative to carbonyl moiety to the mechanism of action against the all of the tested bacteria except E. faecalis and E. coli. The compounds 7a-c have a good spectrum antimicrobial activity against all tested strains.
3 Conclusions
In conclusion, we have described an efficient, clean and simple method for the preparation of pyrido[2,3-d:6,5-d]dipyrimidines, pyrazolo[4’,3’:5,6]pyrido[2,3-d]pyrimidine and indeno[2’,1’:4,5]pyrorolo[2,3-d]pyrimidine derivatives via a cyclo-condensation reaction in water. This protocol includes some important aspects like the use of water as a “green” reaction medium, high atom economy and mild reaction conditions.
4 Experimental
4.1 Materials and techniques
Melting points were taken on an Electrothermal 9100 apparatus and left uncorrected. IR spectra were obtained on a Shimadzu IR-470 spectrometer. 1H and 13C NMR spectra were recorded on a BRUKER DRX-300 AVANCE spectrometer at 300.13 and 75.47 MHz. NMR spectra were obtained on solutions in DMSO using TMS as internal standard. All of the chemicals were purchased from Fluka, Merck and Aldrich and used without purification.
4.2 Typical procedure
4.2.1 1,3-dimethyl-5-phenyl-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4a)
A mixture of 1,3-dimethyl-6-amino-uracil (1 mmol), benzaldehyde (1 mmol), barbituric acid (1 mmol) and p-TSA (0.1 g) in refluxing water (5 ml) was stirred for 4 h (the progress of reaction was monitored by TLC). After completion of reaction, the reaction mixture was filtered and the precipitate washed with water and then EtOH to afford the pure product 4a as a white powder (86%). MP > 350 °C; IR (KBr) v = 3322, 2998, 1709, 1695 cm−1. MS, m/z (%): 353 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.07 (s, 3H, CH3), 3.42 (s, 3H, CH3), 4.77 (s, 1H, CH), 7.09–7.26 (m, 5H, HAr), 8.94 (bs, 1H, NH), 10.11 (bs, 1H, NH), 10.89 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 28.1, 30.1, 34.5, 90.0, 90.1, 126.6, 128.2, 143.8, 146.2, 149.9, 150.8, 160.8, 162.8. Anal. Calcd. for C17H15N5O4: C, 57.79; H, 4.28; N, 19.82%. Found: C, 57.74; H, 4.24; N, 19.86%.
4.2.2 1,3-dimethyl-5-(4-chlorophenyl)-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4b)
White powder; m.p. > 350 °C; IR (KBr) v = 3332, 3147, 2977, 1698 cm−1. MS, m/z (%): 387 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.07 (s, 3H, CH3), 3.39 (s, 3H, CH3), 4.75 (s, 1H, CH), 7.05–7.33 (m, 4H, HAr), 8.95 (bs, 1H, NH), 10.12 (bs, 2H, 2NH), 10.92 (s, 1H, NH). Anal. Calcd. for C17H14ClN5O4: C, 52.65; H, 3.64; N, 18.06%. Found: C, 52.71; H, 3.59; N, 18.00%.
Due to very low solubility of the product 4b, we can not report the 13C NMR data for this product.
4.2.3 1,3-dimethyl-5-(4-nitrophenyl)-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4c)
White powder; m.p. > 350 °C; IR (KBr) v = 3460, 2931, 1694 cm−1. MS, m/z (%): 398 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.07 (s, 3H, CH3), 3.42 (s, 3H, CH3), 4.90 (s, 1H, CH), 7.56 (d, 2H, 3JHH = 9.2 Hz, HAr), 8.07 (d, 2H, 3JHH = 9.1 Hz, HAr), 9.04 (bs, 1H, NH), 10.11 (s, 1H, NH), 10.93 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 28.1, 30.2, 35.2, 88.7, 88.9, 123.4, 128.3, 129.6, 146.4, 149.8, 150.8, 153.7, 154.9, 160.8, 162.8. Anal. Calcd. for C17H14N6O6: C, 51.26; H, 3.54; N, 21.10%. Found: C, 51.30; H, 3.59; N, 21.04%.
4.2.4 1,3-dimethyl-5-(4-metoxyphenyl)-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4d)
White powder; m.p. > 350 °C. IR (KBr) v = 3260, 3013, 1714, 1670, 1609 cm−1. MS, m/z (%): 383 (M+). 1H NMR (300 MHz, DMSO-d6 ): δ = 3.09 (s, 3H, CH3), 3.41 (s, 3H, CH3), 3.67 (s, 3H, CH3), 4.76 (s, 1H, CH), 6.67–7.08 (m, 4H, HAr), 8.93 (bs, 1H, NH), 10.11 (bs, 1H, NH), 10.90 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 28.1, 30.1, 34.4, 55.2,. 89.9, 89.9, 114.7, 120.3, 129.2, 143.8, 147.7, 149.9, 150.8, 159.2, 160.9, 162.9. Anal. Calcd. for C18H17 N5O5: C, 56.39; H, 4.47; N, 18.27%. Found: C, 56.45; H, 4.51; N, 18.22%.
4.2.5 5-phenyl-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4e)
White powder; m.p. > 350 °C; IR (KBr) v = 3235, 3089, 1705, 1663 cm−1. MS, m/z (%): 325 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.73 (s, 1H, CH), 7.01–7.22 (m, 5H, HAr), 9.95 (s, 2H, 2NH), 10.99 (s, 2H, 2NH). Anal. Calcd. for C15H11N5O4: C, 55.39; H, 3.41, N, 21.53%. Found: C, 55.34; H, 3.45; N, 21.47%.
Due to very low solubility of the product 4e, we can not report the 13C NMR data for this product.
4.2.6 5-(4-chlorophenyl)-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4f)
White powder; m.p. > 350 °C. IR (KBr) v = 3265, 3147, 3019, 1719, 1714, 1681 cm−1. MS, m/z (%): 359 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.65 (s, 1H, CH), 7.21–7.24 (m, 4H, HAr), 10.84 (s, 2H, 2NH), 11.15 (s, 2H, 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 33.3, 89.1, 128.1, 130.0, 131.0, 144.0, 145.3, 150.2, 162.9. Anal. Calcd. for C15H10ClN5O4: C, 50.08; H, 2.80; N, 19.47%. Found: C, 50.14; H, 2.85; N, 19.40%.
4.2.7 5-(4-nitrophenyl)-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6,8(1H,3H,5H,7H)-tetraone (4 g)
White powder; m.p. > 350 °C. IR (KBr) v = 3238, 3029, 1724, 1675 cm−1. MS, m/z (%): 370 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.76 (s, 1H, CH), 7.51 (d, 2H, 3JHH = 9.1 Hz, HAr), 8.07 (d, 2H, 3JHH = 9.2 Hz, HAr), 10.86 (s, 2H, 2NH), 11.19 (s, 2H, 2NH). 13C NMR (75 MHz, DMSO-d6): δ = 34.3, 88.5, 128.5, 129.5, 131.5, 144.3, 146.3, 150.3, 162.9. Anal. Calcd. for C15H10N6O6: C, 48.66; H, 2.72; N, 22.70%. Found: C, 48.71; H, 2.68; N, 22.75%.
4.2.8 1,3-dimethyl-5-phenyl-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H,5H,7H)-trione (4 h)
Cream powder; m.p. > 350 °C; IR (KBr) v = 3217, 3189, 1705, 1687 cm−1. MS, m/z (%): 369 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.08 (s, 3H, CH3), 3.42 (s, 3H, CH3), 4.68 (s, 1H, CH), 7.09–7.29 (m, 5H, HAr), 8.96 (bs, 1H, NH), 11.80 (s, 1H, NH), 12.34 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 28.1, 30.1, 34.6, 89.7, 94.3, 126.8, 128.2, 128.3, 143.7, 145.6, 150.8, 160.3, 160.8, 173.8. Anal. Calcd. for C17H15N5O3S: C, 55.27; H, 4.09; N, 18.96%. Found: C, 55.33; H, 4.04; N, 18.90%.
4.2.9 1,3-dimethyl-5-(4-chlorophenyl)-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H, 5H,7H)-trione (4i)
Cream powder; m.p. > 350 °C; IR (KBr) v = 3226, 1706, 1678 cm−1. MS, m/z (%): 403 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.07 (s, 3H, CH3), 3.40 (s, 3H, CH3), 4.78 (s, 1H, CH), 7.23–7.28 (m, 4H, HAr). 9.08 (bs, 1H, NH), 11.47 (s, 1H, NH), 12.34 (s, 1H, NH) Anal. Calcd. for C17H14ClN5O3S: C, 50.56; H, 3.49; N, 17.34%. Found: C, 50.60; H, 4.34; N, 17.27%.
Due to very low solubility of the products 4j–l, we can not report the 13C NMR data for these products.
4.2.10 5-phenyl-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H,5H,7H)-trione (4j)
Yellow powder; m.p. 296 °C dec.; IR (KBr) v = 3276, 3045, 1703, 1678 cm−1. MS, m/z (%): 341 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.80 (s, 1H, CH), 7.19–7.21 (m, 5H, HAr), 9.04 (bs, 1H, NH), 9.75 (bs, 1H, NH), 10.78 (s, 1H, NH), 12.45 (bs, 1H, NH). Anal. Calcd. for C15H11N5O3S: C, 52.78; H, 3.25; N, 20.52%. Found: C, 52.83; H, 3.29; N, 20.60%.
4.2.11 5-(4-chlorophenyl)-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H,5H,7H)-trione (4k)
Yellow powder; m.p. 298 °C dec.; IR (KBr) v = 3273, 3056, 1719, 1658 cm−1. MS, m/z (%): 375 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.62 (s, 1H, CH), 720–7.22 (m, 4H, HAr), 9.03 (bs, 1H, NH), 9.77 (bs, 1H, NH), 10.76 (s, 1H, NH), 11.77 (bs, 1H, NH). Anal. Calcd. for C15H10ClN5O3S: C, 47.94; H, 2.68; N,18.64%. Found: C, 47.88; H, 2.63; N, 18.70%.
4.2.12 5-(4-nitrrophenyl)-8-thioxo-9,10-dihydropyrido[2,3-d:6,5-d]dipyrimidine-2,4,6(1H,3H,5H,7H)-trione (4l)
Yellow powder; m.p. 290 °C dec.; IR (KBr) v = 3280, 3069, 1713, 1690 cm−1. MS, m/z (%): 386 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 4.78 (s, 1H, CH), 7.53 (d, 2H, 3JHH = 9.4 Hz, HAr), 8.08 (d, 2H, 3JHH = 9.3 Hz, HAr), 10.91 (s, 1H, NH), 11.61 (bs, 1H, NH), 12.30 (s, 1H, NH), 12.33 (bs, 1H, NH). Anal. Calcd. for C15H10N6O5S: C, 46.63; H, 2.61; N,21.75%. Found: C, 46.69; H, 2.66; N, 21.82%.
Products 7a–l are known compounds [37] and we don’t report any characterization data for these compounds.
4.2.13 4b,9b-dihydroxy-1,3-dimethyl-1,4b,9b,10-tetrahydroindeno[2’,1’:4,5]pyrorolo[2,3-d]pyrimidine-2,4,5(3H)-trione (9a)
White powder (92%); m.p. 278–280 °C; IR (KBr) v = 3407, 3234, 1728, 1679, 1633 cm−1. MS, m/z (%): 315 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 3.01 (s, 3H, NCH3), 3.18 (s, 3H, NCH3), 5.90 (s, 1H, OH), 6.79 (s, 1H, OH), 7.53–7.91 (m, 4H, HAr), 9.28 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 30.8, 31.1, 83.6, 83.9, 92.9, 123.2, 125.6, 130.6, 134.7, 136.1, 149.8, 151.7, 153.8, 157.8, 197.7. Anal. Calcd. for C15H13N3O5: C, 57.14; H, 4.16; N,13.33%. Found: C, 57.19; H, 4.20; N, 13.27%.
4.2.14 4b,9b-dihydroxy-1,4b,9b,10-tetrahydroindeno[2’,1’:4,5]pyrorolo[2,3-d]pyrimidin-2,4,5(3H)-trione (9b)
White powder (88%); m.p. > 330 °C dec.; IR (KBr) v = 3219, 2862, 1716, 1698 cm−1. MS, m/z (%): 287 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 5.84 (s, 1H, OH), 6.59 (s, 1H, OH), 7.53–7.97 (m, 4H, HAr), 8.72 (s, 1H, NH), 10.07 (s, 1H, NH),. 11.16 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 82.8, 83.6, 93.0, 123.1, 125.8, 130.5, 134.6, 135.9, 149.9, 151.7, 154.5, 159.6, 197.9. Anal. Calcd. for C13H9N3O5: C, 54.36; H, 3.16; N,14.63%. Found: C, 54.40; H, 3.11; N, 14.70%.
4.2.15 4b,9b-dihydroxy-2-thioxo-1,2,3,4b,9b,10-hexahydroindeno[2’,1’:4,5]pyrorolo[2,3-d]pyrimidine-4,5-dione (9c)
White powder (85%); m.p. > 350 °C; IR (KBr) v = 3285, 3166, 2902, 1715, 1658 cm−1. MS, m/z (%): 303 (M+). 1H NMR (300 MHz, DMSO-d6): δ = 5.97 (s, 1H, OH), 6.67 (s, 1H, OH), 7.27–8.03 (m, 4H, HAr), 8.54 (s, 1H, NH), 11.55 (s, 1H, NH), 12.49 (s, 1H, NH). 13C NMR (75 MHz, DMSO-d6): δ = 82.7, 87.8, 92.9, 123.2, 126.1, 130.6, 134.6, 136.1, 149.9, 153.3, 157.3, 176.1, 197.6. Anal. Calcd. for C13H9N3O4S: C, 51.48; H, 2.99; N,13.85%. Found: C, 51.42; H, 2.95; N, 13.77%.
Acknowledgements
We gratefully acknowledge financial support from the Research Council of Shahid Beheshti University.


