1 Introduction
In 2000, Werner et al. reported a new coordination mode for phosphane ligands with the synthesis of the bimetallic Rh(I) complexes A (Fig. 1) in which tertiary R3P derivatives act as bridging ligands [1]. The phosphane ligands are among the most widely used 2-electron donors in coordination chemistry and, prior to Werner's work, they were almost exclusively known as terminal ligands [2]. This seminal discovery was a breakthrough in coordination chemistry, since binucleating ligands potentially allow the synthesis of di- and polynuclear complexes that are of great interest in manifold fields such as catalysis, bio-inorganic and materials sciences [3]. The second family of stable dimers bearing a bridging phosphane moiety was prepared using the 2,5-bis(2-pyridyl)-phosphole ligand B (Fig. 1). In 2001, the PdI-dimer C1 having two identical Pd-P bond lengths was isolated (Fig. 1) [4]. Following this observation, mixed PdI,PtI-complex C2 [5], CuI-dimers such as D1-3 [5,6] and AgI-dimers such as E1-2 [7] featuring bridging phosphole moieties were also prepared (Fig. 1). More recently, it has been shown that phenylbis(pyrid-2-ylmethyl)phosphane ligand can also stabilize Cu(I)- and Ag(I)-bimetallic complexes such as F (Fig. 1) in which the P-atom acts as a bridging P-center [8].
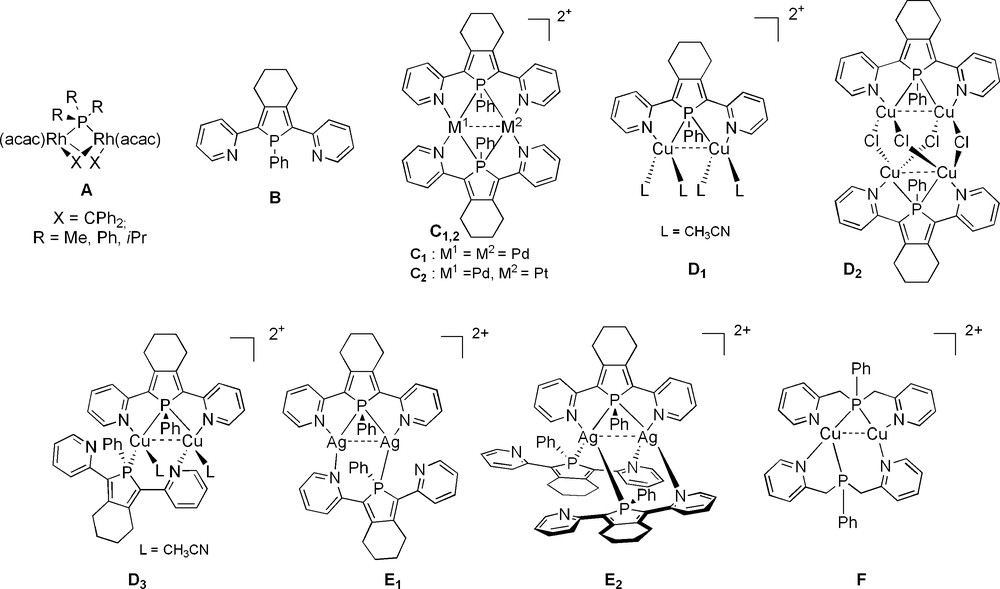
Chemical structure of ligand B and representative examples of metal complexes bearing μ-P atoms.
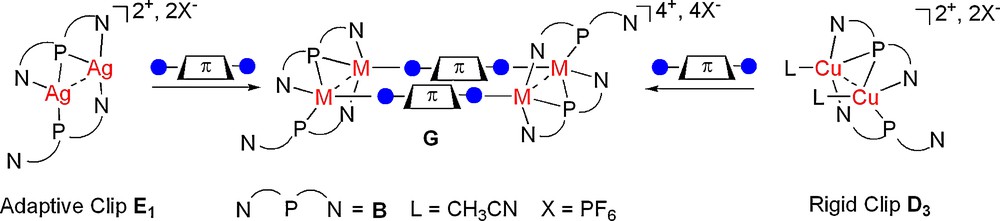
Dimers assembled by the N,P,N-pincer B have found useful utilisation as building blocks in coordination driven supramolecular synthesis; this is an appealing alternative to classic synthetic methodologies for constructing complex molecules. CuI-complex D3 and AgI-complex E1 can be used as ‘rigid’ or ‘adaptative’ bimetallic molecular clips, respectively, for the synthesis of π-stacked metallacycles G upon reaction with linear homoditopic linear π-conjugated linkers (Scheme 1) [9,10]. Numerous supramolecular metallacycles G based on clips bearing a bridging phosphane ligand have been prepared [9,10] demonstrating that this coordination mode is not ‘exotic’ since it allows one to stabilize appealing bimetallic building blocks in supramolecular synthesis.
Herein, we report the synthesis and structural characterization of a novel Cu(I) cluster stabilized by the bis(2-pyridyl)phosphole B in which the P-center acts as a bridging ligand. This [Cu4Cl3] complex is obtained via an unusual route since its [Cu4Cl3] core results from Cl-abstraction from the CH2Cl2 solvent. Moreover, this monocationic derivative, that was characterized in the solid state only due to its isolation in low yield, exhibits an original structure with the μ2-P centres bridging two Cu(I) centres having different coordination numbers and geometries.
2 Characterization of a monocationic [Cu4Cl3] cluster stabilized by two ligands B bearing a bridging phosphane center
A yellow dichloromethane solution of the Cu(I) dimer D1 was exposed to pentane vapour diffusion under air and exposed to daylight at room temperature. After one day, yellow crystals of the derivative D1 started to grow along the wall of the flask and after one week the solution was almost colourless. The complex D1 can be then collected as a yellow polycrystalline material in a very good yield (up to 90–95%) [5,6]. However, if this work-up is conducted for a longer period (2 months) at room temperature in a sealed bottle in order to prevent solvent evaporation, tiny deep red crystals of the new derivative 1 (Fig. 2a) appeared slowly. Only a few crystals were formed preventing NMR characterization or elemental analyses to be performed. However, an X-ray diffraction study (Table 1) performed on a single crystal allowed the molecular structure of this unexpected compound to be established.

(a) Chemical structure of derivative 1; (b) Ortep view of the monocationic complex 1; (c) view of the [Cu4Cl3] core the complex 1; the N,P,N ligands B are schematically represented by the black curved lines.
Crystal data and structure refinement for derivative 1.
| 1· PF6 · 3CH2Cl2 | |
| Molecular formula | C99H88Cl12Cu8F12N8P6 |
| CCDC number | 763502 |
| Molecular weight | 2737.31 |
| a (Å) | 17.481(1) |
| b (Å) | 14.082(2) |
| c (Å) | 21.183(1) |
| α (°) | 90 |
| β (°) | 90.762(1) |
| γ (°) | 90 |
| V (Å3) | 5214.1(8) |
| Z | 2 |
| Dc (g cm−3) | 1.744 |
| Crystal system | Monoclinic |
| Space group | P21/a |
| Temperature (K) | 120(2) |
| Wavelength Mo-Kα (Å) | 0.71069 |
| Crystal size (mm) | 0.3 × 0.3 × 0.3 |
| μ (mm−1) | 2.071 |
| F(000) | 2744 |
| θ limit (°) | 2.66–32.05 |
| Index ranges hkl | −26 ≤ h ≤ 26 |
| −20 ≤ k ≤ 21 | |
| −31 ≤ l ≤ 31 | |
| Reflections collected | 32512 |
| Independant reflections | 18128 |
| Reflections [I > 2σ(I)] | 12825 |
| Data/restraints/parameters | 18128/0/668 |
| Goodness-of-fit on F2 | 1/029 |
| Final R indices [I > 2σ(I)] | R1 = 0.0499 |
| R indices (all data) | R1 = 0.0826 |
| Largest diff peak and hole (e Å−3) | 1.227 and −1.337 |
Derivative 1 crystallizes in the P21/a space group of the monoclinic system. The asymmetric unit contains one monocationic Cu4Cl3(B)2 cluster (Fig. 2b), one PF6− anion and two dichloromethane molecules. The monocation of 1 is based on a highly distorted Cu4 tetrahedron (Fig. 2c). The Cu---Cu distances lie in the range of those usually accepted for cuprophilic interactions between Cu(I) metal centers (Table 2) [6], except for the Cu(2)-Cu(3) distance (2.9540(9) Å), which is markedly longer (Table 2, Fig. 2c).
Selected bond lengths [Å] and angles [°] for complex 1.
| M–μP | M–N | M⋯M | M-Cl | N-M-μP | M-μP-M | μP-M-M | μCl-M-M |
| 2.2867(10) | 2.014(3) | 2.5226(7) | 2.2083(9) | 85.76(7) | 67.15(3) | 56.41(2) | 50.88(2) |
| 2.2875(9) | 2.078(2) | 2.5298(7) | 2.2085(9) | 86.40(7) | 66.99(3) | 56.44(2) | 61.53(2) |
| 2.2574(10) | 2.037(3) | 2.6317(8) | 2.2817(9) | 84.80(7) | 55.45(2) | 49.56(2) | |
| 2.3128(9) | 2.053(2) | 2.6506(8) | 2.3046(9) | 85.65(7) | 57.55(2) | 50.24(2) | |
| 2.7492(8) | 2.4753(10) | 49.67(2) | |||||
| 2.9540(9) | 2.5023(10) | 58.70(3) |
The three μ2-bridging chloride ligands Cl(1), Cl(2) and Cl(3) are linked to the Cu(1) and Cu(3), the Cu(2) and Cu(3), and the Cu(2) and Cu(4) metal centres, respectively (Fig. 1). The Cl(2) anion adopts a classical symmetrically μ2-bridging coordination mode (Δd(Cl(2)-Cu) = 0.023 Å; Cl(2)-Cu-Cu angles, 49.56(2)° and 50.24(2)°). In contrast, the Cl(1) and Cl(3) have a markedly unsymmetrical semi-bridging coordination mode (Δd(Cl(1)-Cu) = 0.294 Å; Cl(1)-Cu-Cu angles, 50.88(2)° and 61.53(2)°; Δd(Cl(3)-Cu) = 0.267 Å, Cl(3)-Cu-Cu angles, 49.67(2)° and 58.70(3)°). The coordination sphere of the Cu(I) metal centre is completed by two ligands B acting as 6-electron μ-1κN:1,2κP:2κN donors with the the P(1) atom bridging the Cu(1) and Cu(3) centers and the P(2) atom bridging the Cu(2) and Cu(4) centers (Fig. 1). The geometric data of the bis(2-pyridyl)phosphole moieties in 1 (Table 3) are unremarkable and compare with those observed in other Cu(I)-complexes [5,6,9].
Selected bonds lengths [Å] and angles [°] of the bis(2-pyridyl)phosphole moieties in the free ligand B and in the derivative 1.
| P-C1 | C1-C2 | C2-C7 | C7-C8 | C1-CPy | C1-P-C8 | |
| P-C8 | C8-CPy | |||||
| B | 1.806(6) | 1.365(9) | 1.478(9) | 1.354(8) | 1.466(9) | 90.5(3) |
| 1.806(6) | 1.467(8) | |||||
| 1 | 1.805(3) | 1.360(4) | 1.459(4) | 1.354(4) | 1.459(4) | 91.19(13) |
| 1.808(3) | 1.461(4) | |||||
| 1.810(3) | 1.358(4) | 1.479(4) | 1.363(4) | 1.457(4) | 90.86(13) | |
| 1.813(3) | 1.460(4) |
If the Cu---Cu interactions are ignored, the Cu(1) and Cu(4) metal centres have a T-shaped trigonal planar coordination sphere, whereas the Cu(2) and Cu(3) metal centres have a distorted tetrahedral geometry (Fig. 2c). It appears that complex 1 features an unprecedented structure in which μ2-bridging phosphane ligands are coordinated to two Cu(I) centres having different (tri- and tetra-) coordination geometries (Cu(1) and Cu(4), ‘P1N1Cl1’ environment; Cu(2) and Cu(3), the ‘P1N1Cl2’ environment, Fig. 2c). Note that in all the other known Cu(I) complexes and the related supramolecular assemblies G [5,6,8], the metal centres always exhibit a tetracoordinated distorted tetrahedron geometry. The only other example of a dimer with a “mixed coordination geometry” involving a μ-P center is the Ag(I) dimer E2 (tetra- and penta-coordinated Ag(I) centres) when associated with the Al[OC(CF3)3]4}− counter-anion [7]. It is striking to note that in a highly unsymmetrical environment (mixed coordination centres in a highly distorted tetrahedral Cu4Cl3 core), the two μ2-P atoms are symmetrically bridging the Cu(I) centres (Δ(P(1)-Cu) = 0.001 Å, P(1)-Cu-Cu angles, 56.41(2)° and 56.44(2)°; Δ(P(2)-Cu) = 0.065 Å, P(2)-Cu-Cu angles, 55.45(2)° and 57.55(2)°). This result shows that there is no direct relation between the symmetry of the P-donor and that of the metal fragments.
It is interesting to compare the cationic complex 1 and the neutral complex D2 (Fig. 1), obtained by reacting ligand B with CuCl in dichloromethane [6], that share structural similarities (Fig. 3). Complex D2 (Fig. 3a) bears a fourth μ2-Cl ligand that formally replaces the uncoordinated PF6− counter-anion of 1 and connects the Cu(1) and Cu(4) metal centres [6]. The gross symmetry of the neutral complex D2 is higher than the symmetry of the monocationnic tetramer 1 (Fig. 3). In D2, the four metal centre coordination spheres are ‘P1N1Cl2’ distorted tetrahedrons. All the μ2-bridging chlorine anions are symmetrically bridging the metal centres (Cu-Cl bond lengths, 2.326(2) – 2.374(1) Å), and only two couples of Cu(I) metal centres (those bridged by the μ2-P atoms) are involved in cuprophillic interactions (d(Cu---Cu) = 2.566(1) Å and 2.598(1) Å; the other Cu(I)---Cu(I) distances being longer than 3.18 Å). In the case of the complex 1 (Fig. 3b), the formal ‘removal’ of one bridging μ2-Cl ligand results in (i) a decrease of the symmetry of the Cu4 central core compared to D2 and (ii) an increase of the number of cuprophilic interactions (Fig. 3, Table 2). Most probably these cuprophilic interactions contribute to the stability of the monocationnic Cu4 cluster 1 whose formation is originally due to the geometric constraints accompanying the bridging phosphane mode of the ligand B.

Comparison between the solid state structure of (a) derivative D2 [6] and (b) derivative 1; in both cases, dichloromethane molecules included in the unit cell are not shown.
The formation of complex 1 deserves a discussion since the only potential source of the μ2-Cl ligands in its Cu4Cl3 core is CH2Cl2, which is used as solvent. These Cl-ligands can be generated by two processes: activation of dichloromethane by the Cu(I) centers of D2 or decomposition of dichloromethane by the sun light. Few examples of chlorine ions abstraction from CH2Cl2 mediated by Cu(I) complexes have been previously described [11] and the mechanism of this halide abstraction remains unclear. On the other hand, it has been shown that near UV irradiation of dichloromethane in presence of Cu(I) salts as catalyst causes its decomposition into HCl, C2H2Cl4 and peroxides [12]. In this latter case, HCl would react with D1 affording derivative 1 bearing μ2-Cl ligands. Since our crystallization experiments were performed in sunlight, these two processes can participate to the formation of complex 1. Considering the long reaction time, it is very probable that the formation of complex 1 implies a very slow trapping reaction of the HCl formed upon CH2Cl2 decomposition by the Cu2(B) moieties of D1.
3 Conclusion
In this work, we describe the solid state structure of a new (Cu)4 cluster 1 in which two ligands B act as a 6-electron μ-1κN:1,2κP:2κN donors with a symmetrically bridging P-donor. Derivative 1 is the first example of a mixed coordination Cu(I) complex assembled by μ-P centers. Interestingly, despite the fact that the Cu4 core of derivative 1 is a highly unsymmetrical distorted tetrahedron, the two μ2-P ligands are symmetrically bridging the metal centres.
4 Experimental part
A CH2Cl2 solution (10 mL) of complex D1 (0.050 g, 0.05 mmol) was stirred for 3 h at room temperature. This solution was transferred in glass tubes that were introduced in a glass bottle filled with pentane. This bottle was sealed and left at room temperature under daily light for two months. After one day, yellow crystals of D1 start to grow in the CH2Cl2 solution along the wall of the glass tubes. After two months, a very few amount of tiny red crystals of 1 are observed on the wall of the glass tube close to the surface of the CH2Cl2 solution and can be collected for X-ray diffraction studies on single crystal.
CCDC reference number 763502 contains the supplementary crystallographic data for derivative 1. These data can be obtained free of charge at http://www.ccdc.cam.ac.uk/conts/retreving.html or from the Cambridge Crystallographic Data Center, 12, Union Road, Cambridge CB2 1EZ, UK; fax: (internat.) + 44 1223 336 033; e-mail: deposit@ccdc.cam.ac.uk.


