1 Introduction
In recent years, the synthesis of xanthenes has been of considerable interest because these compounds have a wide range of biological and pharmaceutical properties such as antiviral [1], antibacterial [2], antiflammatory [3], as well as in photodynamic therapy and as antagonism for the paralyzing action of zoxazolamine [4]. Also, these compounds can be used as dyes [5] in laser technologies [6] and fluorescent material for visualization of biomolecules [7]. A variety of the reagents and catalysts [8–29] have been reported for the preparation of these compounds. However, some of the reported methods suffer from drawbacks such as low yields, prolonged reaction times, the use of toxic solvents, special apparatus and the use of toxic catalysts. Therefore, introduction of new and environmentally benign catalytic methods for the preparation of xanthene derivatives is highly desirable.
Heteropoly acids (HPAs) with Keggin type structure are known to be very active catalysts for both acid-catalyzed and redox reactions [30–32]. In the modern era of catalyst design, heteropoly acids offer a unique opportunity to tune solids at the atomic level since they have well-defined local structures in which the constituent elements can be easily varied. However, these catalysts have drawbacks such as short lifetime under reaction conditions, low surface area (<10 m2/g) and insufficient thermal stability [33,34]. To overcome these disadvantages, many efforts including immobilization of HPA on a support have been devoted. Silica, silica-alumina, alumina, titania, zirconia, resins, polymers, clay, MCM-41 and zeolites have been reported as support for immobilization of these compounds [34–39]. Amongst them, zeolite Y has been employed by several researchers as support for immobilization of HPAs because of its supercage diameter [40,41].
In continuation on our previous works on the encapsulation of catalysts in the zeolites [42–44], here, we describe the application of dealuminated zeolite Y-encapsulated molybdophosphoric acid (MPA-DAZY) in the synthesis of xanthene derivatives under solvent-free conditions by conventional heating and under microwave irradiation (Scheme 1).
2 Experimental
All materials were of the commercial reagent grade and obtained from Merck or Fluka. FT-IR spectra were obtained in the range 400–4000 cm−1 with a Nicolet Impact 400D spectrometer. 1H NMR spectra were recorded on a Bruker-Arance AQS 300 MHz spectrometer. The microwave system used for these experiments includes the following items: micro-SYNTH labstation, complete with glass door, dual magnetron system with pyramid-shaped diffuser, 1000 W delivered power, exhaust system, magnetic stirrer, “quality pressure” sensor for flammable organic solvents, ATCFO fiber optic system for automatic temperature control.
2.1 Preparation of catalyst (MPA-DAZY)
The modified DAZY, as support, was prepared by hydrothermal treatment according to the reported procedure [40]. In order to inhibit the decomposition of MPA by basic Na zeolite, dealumination of NaY zeolite should be carried out under hydrothermal conditions. This not only increases the zeolite stability but also yields a secondary pore system in zeolite matrix for deposition of the large MPA species [35,45].
The method reported by Mukai et al. was used for the synthesis of H3PMo12O40 (MPA) encapsulated into DAZY [40]. In a typical procedure, DAZY (2.0 g) and molybdenum (VI) oxide (7.2 g) were mixed in deionized water (70 mL) and stirred at room temperature for 24 h. Then, phosphoric acid (0.48 g, 85%) was added, and the mixture was stirred for 3 h at 368 K. The synthesized sample (MPA-DAZY) was filtered and dried at 383 K and then individually immersed into hot water (363 K). After agitation for 1 h, the sample was filtered and dried at 363 K. Then, the unreacted species of metal cations in the lattice were exchanged with aqueous 1 M solution of NaCl. Therefore, the sample was suspended in a solution of NaCl (1 M) for 4 h under vigorous stirring. Then, the catalyst was filtered, washed thoroughly with distilled water and dried. The amount of MPA into zeolite Y obtained by dissolving a small amount of sample in hydrofluoric acid and hot concentrated hydrochloric acid, and analyzing the obtained solution using atomic absorption spectroscopy.
2.2 General procedure for synthesis of 14-substituted-14-H-dibenzo[a,j] xanthenes in the presence of MPA-DAZY under thermal and under MW irradiation conditions
2-Naphthol (2 mmol), aldehyde (1 mmol) and catalyst (300 mg, 0.0125 mmol) were mixed and heated at 100 °C or irradiated at 800 W for an appropriate time. The progress of the reaction was monitored by TLC. At the end of the reaction, Et2O (15 mL) was added and MPA-DAZY was filtered. The organic layer was dried over Na2SO4 to give the crude xanthene. The solvent was evaporated and the product was recrystallized from ethanol to yield the pure produce.
3 Results and discussion
3.1 Preparation and characterization of MPA-DAZY
The prepared catalyst was characterized by infrared spectroscopy (FT-IR), X-ray diffraction (XRD), differential thermal gravimetry (DTG) and elemental analysis techniques.
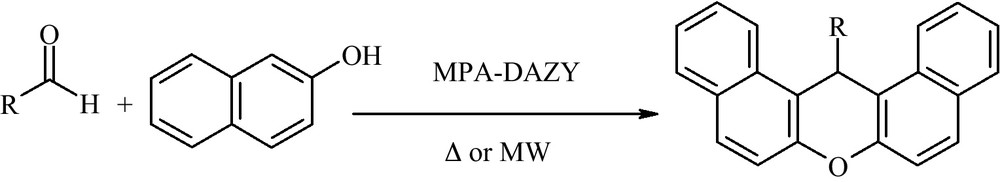
The content of MPA in the synthesized sample, which was measured based on the Mo content of the MPA-DAZY, was obtained 0.0875 g (0.043 mmol) per gram of the supported catalyst. The FT-IR spectra confirm the existence of MPA in the zeolite matrix. In the IR spectra of MPA, six characteristic peaks are observed at 1627, 1063, 963, 871, 782, 595 cm−1. When the MPA is encaged into USY zeolite, these peaks are observed at 1634, 1412, 1043 (PO), 919 (MoOt), 724 (MoOeMo) (Ot: terminal oxygen, Oe: edge-sharing oxygen) 564 cm−1. The shift in the position of peaks reveals that the Keggin structure interacts with Y zeolite mostly through corner-shared [40,46] (Fig. 1). Since all of the MPA, which have been absorbed on the surface, was washed away in the washing process; therefore, the presence of MPA bands in the IR spectra clearly proves the presence of MPA in the zeolite cages.
FT-IR spectra of: (A) DAZY; (B) MPA and (C) MPA-DAZY.
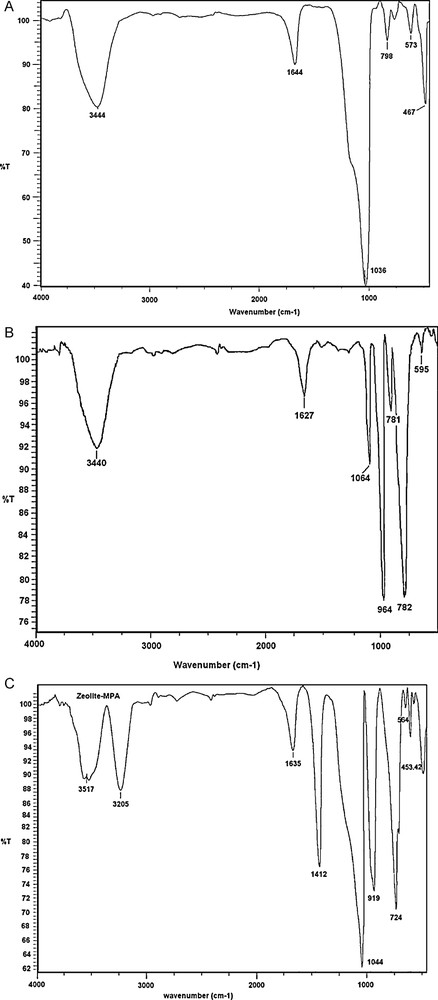
The XRD patterns indicated that the calcinated Y zeolite and MPA-DAZY have crystallinity almost identical to the parent NaY zeolite. Upon calcination of NaY zeolite, the intensity of the main peaks of the zeolite only slightly reduces, which indicates that the framework of zeolite is still maintained after dealumination of zeolite by hydrothermal treatment. In the XRD pattern of MPA-DAZY, the disappearance of the corresponding peaks of pure H3PMo12O40 (2θ = 7.2) proves the encapsulation of Keggin structure in the dealuminated zeolite matrix [32,46,47] (Fig. 2).
XRD patterns of: (A) NaY zeolite; (B) calcinated NaY zeolite; (C) HMP-DAZY and (D) MPA.
Thermogravimetric analysis showed that when the MPA is formed into supercage of zeolite, the thermal stability of MPA increases and the decomposition temperature of MPA-DAZY is higher than pure MPA. This behavior can be attributed to the difficulty of O2 access inside the supercage of zeolite for decomposition of MPA. Meanwhile, the strong interaction between MPA and zeolite framework through corner-shared can confirmed this occurrence [48].
3.2 Synthesis of xanthenes catalyzed by MPA-DAZY under thermal conditions
First, the amount of catalyst was optimized in the condensation reaction of 2,4-dichlorobenzaldehyde (1 mmol) with 2-naphthol (2 mmol) under conventional heating at 100 °C. The results showed that the highest yield obtained in the presence of 0.0125 mmol (0.3 g) of catalyst, whereas the yield of the product is reduced by decreasing the amount of the catalyst or decreasing the temperature. When the same reaction was performed in the absence of the catalyst, the corresponding product was obtained in only 5% yield.
The dependency of MPA amount in the catalysts on the catalytic activity was also investigated. In this manner, catalysts with different MPA loading were prepared and used in the conversion of 4-nitrobenzaldehyde to its corresponding xanthene. The results showed that the catalyst with 0.043 mmol/g of catalyst was more efficient than the others (Table 1).
The effect of catalyst loading in synthesis of 14-substituated-14H-dibenzo[a,j] xanthenes under conventional heatinga.
| Entry | Catalyst loading (mmol/g) | Time (min) | Yield(%)b |
| 1 | 0.025 | 110 | 45 |
| 2 | 0.03 | 110 | 60 |
| 3 | 0.043 | 110 | 93 |
| 4 | 0.055 | 110 | 93 |
a Reaction conditions: 4-nitrobenzaldehyde (1 mmol), β-naphthol (2 mmol), catalyst (0.3 g).
b Isolated yield.
Under the optimized reaction conditions, a wide range of aromatic aldehydes with electron-withdrawing and electron-donating substituents were reacted with 2-naphthol in the presence of 0.0125 mmol (0.3 g) of MPA-DAZY under solvent-free conditions to afford the corresponding dibenzoxanthene derivatives in high yields (Table 2, entries 1–13).
MPA-USZY catalyzes the synthesis of 14-substituated-14H-dibenzo[a,j] xanthenes under conventional heating and microwave irradiationa.
| Entry | Aldehyde | Xantene | |||
| Thermal conditions | Microwave irradiation | ||||
| Yield (%)b,c | Time (min) | Yield (%)b,c | Time (min) | ||
| 1 | 85 | 120 | 95 | 3 | |
| 2 | 75 | 80 | 90 | 2 | |
| 3 | 95 | 120 | 78 | 4 | |
| 4 | 98 | 120 | 93 | 3 | |
| 5 | 95 | 90 | 95 | 3 | |
| 6 | 87 | 80 | 88 | 3 | |
| 7 | 93 | 110 | 92 | 6 | |
| 8 | 88 | 130 | 82 | 4 | |
| 9 | 93 | 120 | 85 | 6 | |
| 10 | 78 | 120 | 88 | 7 | |
| 11 | 90 | 130 | 82 | 4 | |
| 12 | 86 | 150 | 90 | 35 | |
| 13 | 95 | 90 | 93 | 6 |
a Reaction condition: aldehyde (1 mmol), β-naphthol (2 mmol), catalyst (0.3 g, 0.0125 mmol).
b Isolated yield.
c The products were identifide by m.p. and IR and NMR spectroscopic data.
3.3 Synthesis of xanthenes catalyzed by MPA-DAZY under MW irradiation
The synthesis of xanthenes was also investigated in the presence of MPA-DAZY under microwave irradiation. The highest yield of the product was obtained at the power of 800 W. At this power, the temperature of the reaction vessel was measure to be 40 °C for liquid aldehydes and 70 °C for solid aldehydes.
Under the optimized reaction conditions, different aromatic aldehydes were reacted with 2-naphthol in the presence of this catalyst under microwave irradiation to afford the corresponding xanthene in high yields (Table 2). The results showed that the reaction times are considerably shorter under microwave irradiation. The acceleration of reactions by microwave mainly results from two effects: thermal effect and a specific microwave effect. Thermal effect (dielectric heating) resulting from material–microwave interaction, which allows fast and uniform distribution, and conversion of microwave energy into heat.
A specific microwave effect can be expected for the polar mechanism, when the polarity is increased during the reaction from the ground state (GS) towards the transition state (TS) [49,50].
When HY zeolite was used as support, the same results were obtained. These results were expected because NaY zeolite is converted to HY zeolite during the dealumination process [40].
3.4 Catalyst recovery and reused
The reusability of the encaged MPA-DAZY, both under thermal conditions and under MW irradiation, was tested using 4-nitrobenzaldehyde as a model substrate. At the end of the reaction, the catalyst was filtered and activated by washing with ethyl acetate and drying at 120 °C for 3 h. No appreciable loss in the catalytic activity was observed, which means that MPA is still present in the supercages of zeolite. The filtrates were used for measuring the amount of Mo leached by ICP. The results, which are summarized in Table 3, showed that only small amounts of initial Mo is leached in three first runs and in the fourth run, no Mo is leached. The yields of corresponding xanthene after fourth run were 85% and 87% under thermal conditions and under MW irradiation, respectively.
The results obtained in the reusability of HMP-DAZY catalyst recovery and the amount of molybdenum leached in the formation of xanthenes from 4-nitrobenzaldehydea.
| Run | Microwave irradiation | Thermal conditions | ||||
| Time (min) | Yield (%)b | Mo leached (%)c | Time (min) | Yield (%)b | Mo leached (%)c | |
| 1 | 120 | 93 | 2 | 3 | 92 | 1.8 |
| 2 | 120 | 87 | 1 | 3 | 90 | 1.0 |
| 3 | 120 | 86 | 1 | 3 | 88 | 0.9 |
| 4 | 120 | 85 | 0 | 3 | 87 | 0 |
a Reaction condition: 4-nitrobenzaldehyde (1 mmol), β-naphthol (2 mmol), catalyst (0.3 g, 0.0125 mmol).
b Isolated yield.
c Determined by ICP.
4 Conclusions
In conclusion, MPA-DAZY was used as an efficient catalyst for the synthesis of 14-substituted-14-H dibenzo[a,j] xanthenes derivatives by condensation of various aromatic aldehydes with β-naphthol using MPA-DAZY as a heterogeneous catalyst under solvent-free conditions both under thermal conditions (100 °C) and under microwave irradiation (800 W).
This protocol can be considered as a green catalytic system for efficient preparation of xanthene derivatives. On the other hand, the catalyst can be reused several times without significant loss of its catalytic activity.
Acknowledgements
The financial support of this work by the Strategies Research Council of New Technologies of Isfahan Province is acknowledged.


