1 Introduction
The world consumption of polymers amounts now to 176 Mt and increases annually by 5%. Light and temperature are the main initiators of polymer degradation. They contribute to the formation of free radicals that react with oxygen to form carboxyl or ketone groups during oxidation [1]. The ageing processes of materials bring about irreversible changes in structural properties, which is undoubtedly an undesirable effect [2,3]. In order to establish a specified working life of a product it is indispensable to properly select effective stabilizers, whose basic task will be its protection against oxidation processes. The studies on the photo-stabilization of polymeric composites have moved from the discovery of new stabilizers towards the modification of known structures or the preparation of more effective photo-stabilizing forms [4,5]. From the available literature data, it follows that nowadays the industry uses anti-ageing substances in the form of derivatives of aromatic amines, amines with steric hindrance known as HALS, phosphoric derivatives and substituted phenols [6]. However the 21st century requires the ecosystem to be developed through the elimination of hazardous to the environment chemicals and their replacement by natural, environmentally friendly additives. From the literature, it follows that one should extend the studies on phenol derivatives or naphthquinones [7–10]. Beside the patent of the Goodyear Tire and Rubber Company [11], there is a lack of information about the use of flavonoides as anti-ageing substances in elastomers. This would indicate that our studies are of an innovative character. Based on the analysis of the degradation mechanism of elastomers, we can conclude that the incorporation of natural anti-oxidants such as flavonimide derivatives into these rubbers can improve the stabilization of these materials [12–14].
The ageing process of polymers proceeds through the formation of active radical forms responsible for oxidation processes. From the literature, it follows that flavonoides are very good reducing agents, so they can react with the reactive forms of oxygen or other oxidants as well as they can form complexes with transient metals [15–17]. Thus, it seems justified to use flavonoide derivatives as anti-ageing substances. Such a solution seems to be both ecological and innovatory [16].
2 Materials and methods
2.1 Reagents
The object of study was ethylene-propylene rubber (EPM, Dutral CO-054, manufacturer: Montedison Ferrara – Italy). Dicumyl peroxide, DCP (from Fluka) was used as cross-linking agent, 1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (Sigma Aldrich Chemie GmbH) as co-agent of cross-linking and hexadecyltrimethylammonium bromide, CTBA (Sigma Aldrich Chemie GmbH) as dispersing agent. Areosil 380 silica (from Degussa) was used as filler.
The anti-ageing substances used included the following flavonoide derivatives: Xanthone (Fluka 97%), Naringin (Fluka 95%), Flavone (Aldrich 98%), Morin hydrate (Fluka 98%), Rutin (Sigma 95%), 4,5,7-Trihydroxyflavone (Aldrich 98%), Hesperidin (Fluka 90%) (Fig. 1). Their efficiency was compared with a commonly used HALS stabilizer Chimassorb81 (Ciba).
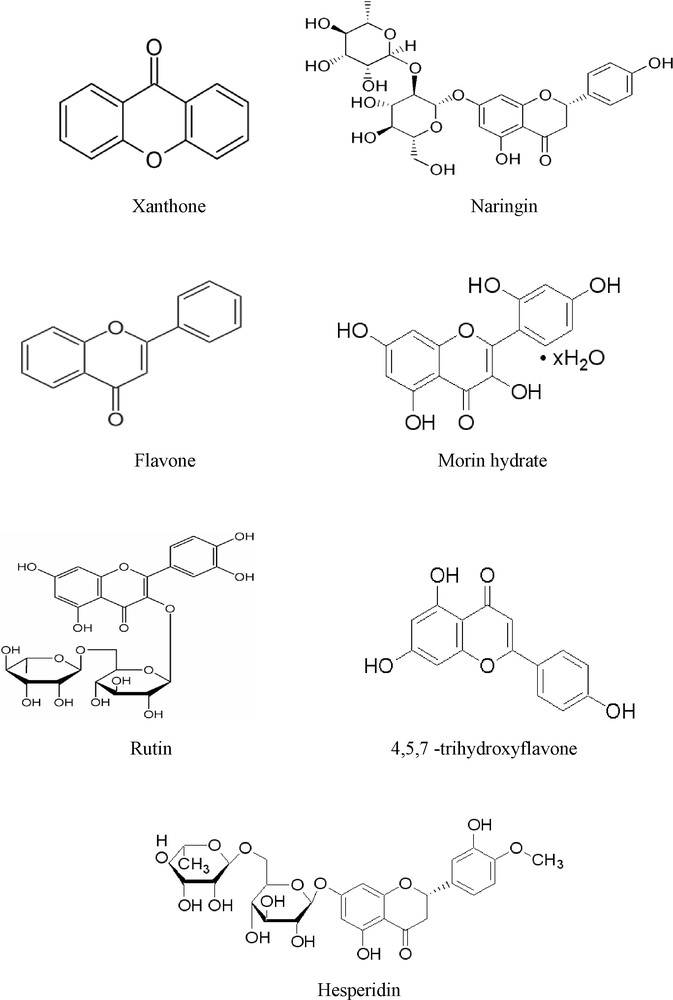
Structures of different flavonoids.
The compositions of elastomer blends are given in Table 1.
Composition of EPM elastomer blends containing flavonoide derivatives.
| Composition | M1 (phr) | M2 (phr) | M3 (phr) | M4 (phr) | M5 (phr) | M6 (phr) | M7 (phr) | M8 (phr) | M9 (phr) |
| EPM | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| DCP | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| CTAB | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 | 2.00 |
| 1,3,5-Triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| A380 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 | 30 |
| Chimassorb81 | 1.0 | ||||||||
| Xanthone | 1.0 | ||||||||
| Naringin | 1.0 | ||||||||
| Flavone | 1.0 | ||||||||
| Morin hydrate | 1.0 | ||||||||
| Rutin | 1.0 | ||||||||
| 4,5,7-Trihydroxyflavone | 1.0 | ||||||||
| Hesperidin | 1.0 |
2.2 Measurement methods
Rubber blends were prepared by means of a laboratory mixing mill with rolls of the following dimensions: length L = 330 mm, diameter D = 140 mm. The speed of rotation of the front roll was Vp = 20 rpm, friction 1.1, the average temperature of rolls was about 40 °C.
The vulcanization of rubber blends was carried out with the use of steel vulcanization molds placed between the shelves of electrically heated hydraulic press. A teflon film was used as spacers preventing the adherence of blends to the press plates. Samples were vulcanized at a temperature of 160 °C, under a pressure of 15 MPa for 30 min.
The density of crosslinks in the vulcanizates network was determined by the method of equilibrium swelling according to standard PN-74/C-04236. The vulcanizates were subjected to equilibrium swelling in toluene for 48 h at room temperature. The swollen samples were then weighed on a torsion balance and dried in a dryer at a temperature of 60 °C to a constant weight and after 48 h they were reweighed. The cross-linking density was determined on the basis of Flory-Rehner's equation:
| (1) |
The tensile strength of vulcanizates was tested according to standard PN-ISO 37:1998 by means of a ZWICK tester, model 1435, for dumbbell w-3.
Ageing characteristics were determined according to standard PN-82/C-04216. Samples were subjected to the action of air at elevated temperature (353 K) for 7 days in a dryer with thermo-circulation. UV ageing was performed by means of an UV 2000 apparatus from Atlas. The measurement lasted for 120 h and consisted of two alternately repeating segments with the following parameters: daily segment (radiation intensity 0.7 W/m2, temperature 60 °C, duration 8 h), night segment (no UV radiation, temperature 50 °C, duration 4 h). The ageing coefficient was calculated according to the relationship: S = [TS’*EB’]/[TS*EB], where TS: tensile strength, EB: elongation at break, TS’, EB’: corresponding values after ageing. The color of the vulcanizates obtained was measured by means of a CM-3600d spectrophotometer. The radiation source consisted of four impulse xenon tubes. The spectral range of the apparatus was 360–740 nm, where change of color ΔE was calculated by the equation:
| (2) |
The spectra of vulcanizates were taken by means of a Bio-Rad IR spectrophotometer. The carbonyl index was calculated on the basis of the ratio of the dependence of band at a wavelength of ∼1700 cm−1 (carbonyl group) to the intensity of band at ∼2800 cm−1 (–CH group).
3 Results and discussion
3.1 Effect of flavonoids on the cross-linking density of EPM vulcanizates
The incorporation of flavonoides into ethylene-propylene rubber brought about a decrease in the density of cross-linking in comparison to that of EPM vulcanizate containing no anti-oxidants. This trend was observed in all the EPM vulcanizates containing flavonoide anti-oxidants, as well as commonly used stabilizers. The lowest cross-linking density was shown by the EPM vulcanizate containing flavone. This testifies to the reaction between anti-oxidants and the radical formed from the decomposition of DCP. UV ageing brought about a considerable increase in the density of crosslinking of the EPM vulcanizates containing flavonoides. Only in the case of EPM vulcanizates without antioxidants and EPM vulcanizate containing hydroxymorin, was the density of crosslinking decreased due to degradation processes. One of the ageing forms is cross-linking taking place by recombination of free macro-radicals into branched structures. Therefore the increase in the cross-linking density of most EPM vulcanizates is connected with the cross-linking processes under the influence of elevated temperature and UV radiation.
An increase in the cross-linking density was observed in the case of EPM vulcanizate containing no anti-oxidant. The residual excess of dicumyl peroxide brought about additional cross-linking of EPM vulcanizate under the influence of elevated temperature. An exception is the sample containing hydroxymorin that shows a slight drop in cross-linking in comparison with the sample not subjected to ageing. The highest cross-linking density, amounting to 10.7 × 105 mol/cm3, was shown by the EPM vulcanizate with xanthone (Table 2).
Effect of flavonoids on the cross-linking density of EPM vulcanizates.
| Vulcanizates | ν(t)*105, (mol/cm3) | ν(t)*105, (mol/cm3) | ν(t)*105, (mol/cm3) |
| Before ageing | After UV ageing | After thermal ageing | |
| EPM | 6.10 | 2.92 | 9.30 |
| EPM/chimassorb81 | 3.53 | 3.74 | 3.86 |
| EPM/xanthone | 4.16 | 4.92 | 10.70 |
| EPM/naringin | 4.24 | 7.53 | 9.36 |
| EPM/flavone | 3.39 | 4.21 | 6.29 |
| EPM/morin hydrate | 3.86 | 2.14 | 3.14 |
| EPM/rutin | 3.90 | 4.34 | 5.35 |
| EPM/4,5,7-trihydroxyflavone | 3.84 | 4.26 | 8.96 |
| EPM/hesperidin | 4.46 | 5.47 | 10.1 |
3.2 Effect of flavonoids on the tensile strength of EPM vulcanizates after ageing
Based on the test results, it has been found that the addition of flavonoides increases the tensile strength of the vulcanizates under investigation. The sample containing rutin showed however a slight increase in the value of TS in comparison to that of the vulcanizate without anti-oxidants (Table 3). The rutin-containing sample was distinguishable from all the vulcanizates subjected to UV ageing by its decreased tensile strength, while the sample with flavone showed a decreased elongation at break. Based on the thermal ageing coefficients calculated from the ratio of the sample deformation energy before after ageing to the sample deformation energy after ageing, it has been found that the hydroxymorin-containing vulcanizate is mostly affected by the ageing process because hydroxymorin has no carbohydrate groups and a lower number of hydroxyl groups, which is connected with a lower anti-oxidizing yield in comparison to the remaining flavonoides. From the calculated proportions of elongation at vulcanizate break after ageing to that before ageing it follows that the highest increase in elongation takes place in the vulcanizate containing trihydroxyflavone or hesperidin. The best protection against thermal ageing was provided by naringin and the commercial antioxidant Chimassorb81, as indicated by the ageing coefficient of EPM vulcanizate amounting to 0.96 and 0.92, respectively (Table 4). As the number of –OH groups increases in flavonoides one can observe a trend towards a slower oxidation, which is illustrated by naringin possessing a developed structure with numerous hydroxyl groups [17].
Effect of flavonoids on the tensile strength of EPM vulcanizates.
| Vulcanizates | SE300, (MPa) | TS, (MPa) | Eb, (%) |
| EPM | 2.91 | 18.53 | 754 |
| EPM/chimassorb81 | 2.18 | 14.70 | 725 |
| EPM/xanthone | 2.73 | 17.08 | 769 |
| EPM/naringin | 2.59 | 12.78 | 757 |
| EPM/flavone | 2.02 | 11.89 | 887 |
| EPM/morin hydrate | 1.36 | 18.53 | 943 |
| EPM/rutin | 1.79 | 20.04 | 871 |
| EPM/4,5,7-trihydroxyflavone | 2.51 | 19.98 | 760 |
| EPM/hesperidin | 2.70 | 17.02 | 741 |
Mechanical properties of the EPM vulcanizates containing flavonoides subjected to thermal ageing.
| Vulcanizates | SE300, (MPa) | TS, (MPa) | Eb, (%) | TSA/TSBA | EbA/EbBA | (TS*Eb)A/(TS*Eb)BA |
| After Thermal ageing | ||||||
| EPM | 2.95 | 12.14 | 705 | 0.66 | 0.94 | 0.61 |
| EPM/chimassorb81 | 1.89 | 12.80 | 769 | 0.87 | 1.06 | 0.92 |
| EPM/xanthone | 1.99 | 15.19 | 927 | 0.89 | 1.21 | 1.07 |
| EPM/naringin | 2.75 | 13.41 | 694 | 1.05 | 0.92 | 0.96 |
| EPM/flavone | 2.73 | 9.43 | 540 | 0.79 | 0.61 | 0.48 |
| EPM/morin hydrate | 1.39 | 8.54 | 905 | 0.46 | 0.96 | 0.44 |
| EPM/rutin | 1.91 | 11.45 | 803 | 0.57 | 0.92 | 0.53 |
| EPM/4,5,7-trihydroxyflavone | 2.34 | 19.42 | 975 | 0.97 | 1.28 | 1.25 |
| EPM/hesperidin | 2.85 | 14.45 | 920 | 0.85 | 1.24 | 1.05 |
The ageing under the influence of UV radiation and elevated temperature has brought about a decrease in the stress at a relative elongation of 300%. The UV ageing coefficients calculated show that the most degraded EPM vulcanizate is that without anti-ageing substances, while the best protection against UV radiation is provided by rutin (Table 5).
Mechanical properties of EPM vulcanizates containing flavonoides, subjected to UV ageing.
| Vulcanizates | SE300, (MPa) | TS, (MPa) | EB, (%) | TSA/TSBA | EBA/EBBA | (TS*EB)A/(TS*EB)BA |
| After UV ageing | ||||||
| EPM | 3.62 | 4.55 | 370 | 0.25 | 0.49 | 0.12 |
| EPM/chimassorb81 | 1.80 | 10.40 | 760 | 0.71 | 1.05 | 0.74 |
| EPM/xanthone | 2.43 | 6.93 | 690 | 0.41 | 0.90 | 0.36 |
| EPM/naringin | 2.61 | 14.22 | 790 | 1.11 | 1.04 | 1.16 |
| EPM/flavone | 1.57 | 10.08 | 855 | 0.85 | 0.96 | 0.82 |
| EPM/morin hydrate | 1.14 | 8.52 | 878 | 0.46 | 0.93 | 0.43 |
| EPM/rutin | 1.47 | 16.61 | 950 | 0.83 | 1.09 | 0.90 |
| EPM/4,5,7-trihydroxyflavone | 1.51 | 13.25 | 856 | 0.66 | 1.13 | 0.75 |
| EPM/hesperidin | 2.84 | 11.97 | 779 | 0.70 | 1.05 | 0.74 |
The colorimetric measurements performed indicate that the color of EPM vulcanizates is changed after each ageing process. Coefficient dE*ab defines to what extent the color of vulcanizate is changed under the influence of a degrading medium.
In addition to their anti-oxidative function, flavonoides play also the role of dyes. The action of UV radiation or elevated temperature considerably changes the vulcanizates. After thermal ageing the most changed color was observed in the hesperidin-containing vulcanizate, while after UV ageing such an effect was observed in the rutin-containing vulcanizate. Based on the colorimetric results obtained, one can also conclude that UV radiation brings about a greater change in color than elevated temperature itself. The change in color under the influence of ageing may indicate the instability of flavonoides (Table 6).
Color parameters of EPM vulcanizates containing flavonoides after UV ageing.
| Vulcanizates | dE*ab | dE*ab |
| After thermal ageing | After UV ageing | |
| EPM | 1.15 | 3.95 |
| EPM/chimassorb81 | 8.81 | 5.31 |
| EPM/xanthone | 0.94 | 5.23 |
| EPM/naringin | 0.99 | 5.76 |
| EPM/flavone | 2.06 | 4.36 |
| EPM/morin hydrate | 2.06 | 0.77 |
| EPM/rutin | 1.22 | 5.38 |
| EPM/4,5,7-trihydroxyflavone | 1.07 | 3.13 |
| EPM/hesperidin | 2.85 | 5.00 |
IR spectroscopic measurements allowed us to determine the carbonyl index on the basis of the proportion of the band intensity derived from carbonyl group to the band intensity derived from C-H group.
The FT-IR method used by us made it possible to determine only the progress of degradation on the surface of material. The highest value of the index was obtained for the EPM vulcanizate containing trihydroxyflavone, while the lowest one for EPM vulcanizate without any addition of anti-ageing substances. The best protection of vulcanizate against ageing was provided by hesperidin (Table 7).
Carbonyl index of EPM vulcanizates containing flavonoides.
| Vulcanizates | Carbonyl index after thermal ageing |
| EPM | 0.13 |
| EPM/chimassorb81 | – |
| EPM/xanthone | 1.25 |
| EPM/naringin | – |
| EPM/flavone | 0.18 |
| EPM/morin hydrate | 0.15 |
| EPM/rutin | – |
| EPM/4,5,7-trihydroxyflavone | 0.20 |
| EPM/hesperidin | 0.16 |
4 Conclusions
Anti-oxidants affect the cross-linking density of ethylene-propylene rubber. Some of peroxide radicals react with flavonoides. This concerns naringin to the lowest extent. During thermal ageing, the cross-linking density of vulcanizates is increased and the most visible changes concern the samples containing xanthone and naringin. The highest degree of degradation is shown by the hydroxymorin-containing vulcanizate, whose coefficient of thermal ageing is equal to 0.44. Thus this compound protects vulcanizates against thermal ageing to the lowest extent. On the other hand, the naringin-containing vulcanizate has a thermal ageing coefficient of 0.96. Hence it is clear that naringin provides the best protection against the negative effect of temperature, similarly to the activity of a commercial and commonly used HALS stabilizer. The anti-oxidative capability of flavonoides can be closely associated with their structure, namely their tendency to reduction in electro-chemical reactions is increased with the increase in the number of hydroxyl groups [17].
In addition to their anti-oxidative effects, flavonoides play also the role of pigments, when added to EPM rubbers they bring about vulcanizate coloration.
The least visible color change takes place in the xanthone-containing vulcanizate. A sample without any anti-oxidant changes its color slightly. The majority of vulcanizates show increased densities of crosslinking. Samples containing no anti-ageing compounds and the hydroxymorin-containing sample subjected to UV ageing show a considerable decrease in the density of cross-linking. The tests of mechanical properties of samples subjected to UV ageing show how powerful are its effects that deteriorate the mechanical characteristics of vulcanizates to a large extent. The least protected vulcanizates are those, which contain hydroxymorin and xanthone and that one without any anti-oxidant.
The best protection of vulcanizates against UV radiation is provided by rutin, where the ageing coefficient is equal to 0.9 indicating insignificant changes in the deformation energy under the influence of UV radiation. The naringin-containing vulcanizate subjected to UV ageing shows the most visible change in color, while the vulcanizate containing hydroxymorin treated with UV radiation changes its color to the lowest extent.
Based on the analysis of IR spectra, it can be stated that in the vulcanizate not subjected to the ageing process, the bands at 1700 cm−1 corresponding to carboxyl groups have a low intensity.
The highest band derived from carboxyl groups is shown by the xanthone-containing vulcanizate.
Undoubtedly, the derivatives of flavonoides fulfill the function of anti-ageing substances in elastomers and at the same time they can replace the carcinogenic stabilizers being now in use on a commercial scale. However, further studies on the anti-ageing effectiveness of flavone derivatives in polymers are still of importance. Xanthone and flavone were oxidised most easily and most quickly, as indicated by their low half-wave potentials and the high rate constants.
The natural anti-oxidant flavonoids, derived from natural sources such as vegetables and fruits, exhibited very high reduction activity in the oxidation process. This confirmed their known anti-oxidant properties, allowing them to be successfully used as anti-ageing substances.
Based on the importance of the intermediate oxidation products and their controllability by electrochemical and selective methods (e.g., under potentiostatic conditions), further study of these compounds is required to fully achieve their potential applicability in both ageing and therapeutic processes.
Acknowledgement
This study was supported by Ministry of Science of Higher Education.


