1 Introduction
Mosquitoes are implicated every year in large outbreaks of severe epidemic diseases around the world. Three hundred and fifty to five hundred million cases of malaria, which is transmitted by Anopheline mosquitoes, are reported annually and over one million people die, most of them young children in sub-Saharan Africa [1,2]. Prevention and control rely on reducing the number of infected people. A personal protection using repellent products is necessary to minimize the risk of infection and reduce the discomfort caused by mosquitoes. Repellents often contain N,N-diethyl-m-methylbenzamide (DEET), ethyl 3-[(acetyl)(butyl)amino]propanoate (IR3535), Icaridin (RS)-sec-butyl-(RS)-2-(2hydroxyethyl)piperidine-1-carboxylate, para-Menthane-3,8-diol (PMD) as synthetics actives [3–5]. However, these synthetic active molecules are often prone to controversial matters due to their potential toxicity. Despite its great protection efficacy, DEET for instance, has been implicated in severe neurotoxicity factors in humans [6–8]. Qui et al. [9] concluded that DEET exhibits a good margin of safety but does manifest some adverse effects. The use of natural repellents is more and more supported by Federal governments or local authorities and demonstrated over the years their potential to replace DEET or others synthetics active, which are active against some mosquito species [10–13]. The use of essential oils extracted from the seeds, the leaves, the branches, the resin of trees or plants as active in repellent formulation is a main interest especially for local market target. Eucalyptus citriodora essential oil is for instance predominately produced in subtropical or tropical countries where, mainly diseases transmitted by mosquitoes (malaria, dengue…) occur [14].
Essential oils possess complex compositions with unique biological activities and have demonstrated, in the past, good efficacy against mosquito [15]. Nevertheless, they often showed a short lasting protection and are usually excluded from repellent products as main active [16]. To extend their efficacy and to guarantee the highest protection, two ways are considered. On the one hand, combinations of different essentials oils are employed and then integrated in a proper formulation to prolong the repellent action over time. On the other hand, the pure essential oil might be used as a starting material in a synthesis process to produce new actives with a higher and longer protection efficacy [17].
In the present case, PMD, a well-known active to repel mosquitoes [18], was synthesized from E. citriodora oil. It has been found in the past that PMD is easily obtained from (+)-citronellal (1), which is the main component (74%) in the E. citriodora oil [19]. The cis and trans PMDs isomers (2a-2b-2c-2d) are produced through an acid-catalyzed cyclisation (Prins reaction) of (+)-citronellal as shown on Scheme 1, by a treatment of a citric acid aqueous solution [20].
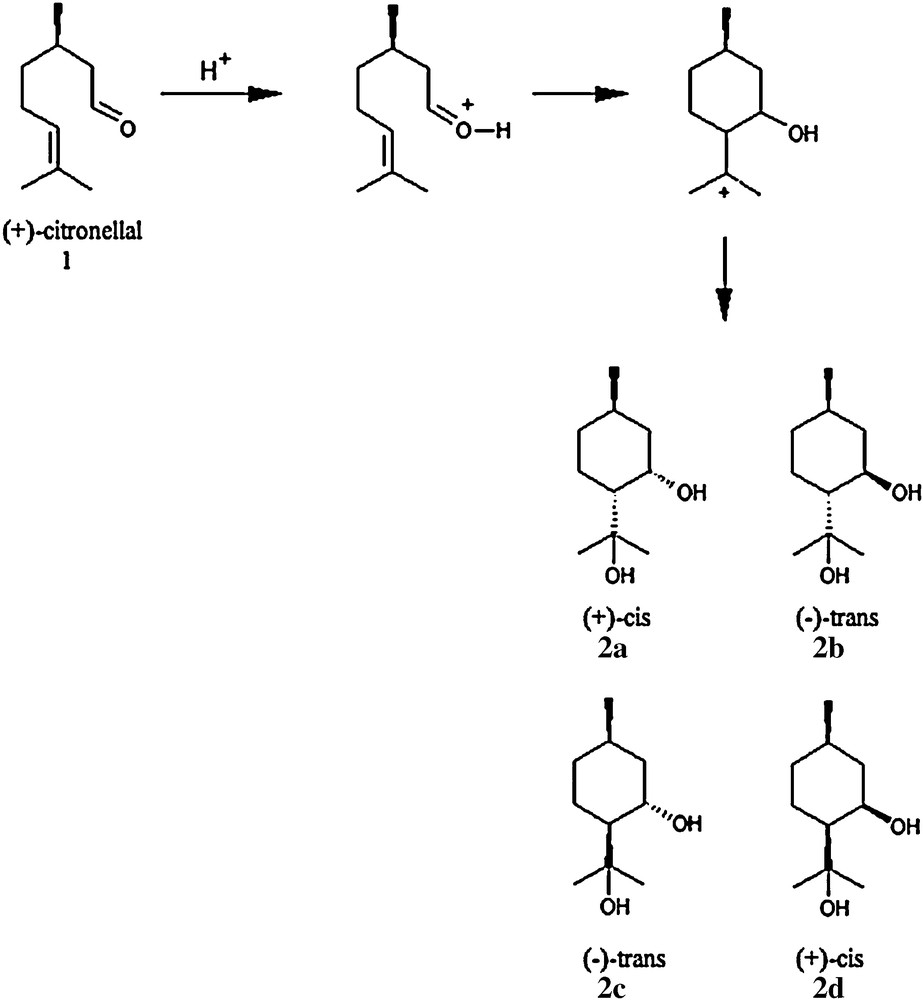
Acid-catalyzed cyclisation of (+)-citronellal. Synthesis of cis and trans-(±)-para-Menthane-3,8-diol.
Also, the use of citric acid during the synthesis leads to the production of a natural PMD as long as citric acid is made from a complete biological and natural process. Investigations on different parameters (acid concentration, reaction time and temperature, quantity of H2O in the medium) were performed to reach the best conversion of (+)-citronellal and the highest selectivity of PMD during the reaction. Gas chromatography analyses were performed to identify and quantify each chemical in the new mixture medium.
The modified oil was then subjected to a repellent test on human volunteers by using on a bioassay Aedes aegypti mosquitoes. Investigations involved the use of DEET as well as pure PMD and pure E. citriodora essential oil as a test comparison with the new reaction mixture.
Finally, thermogravimetric analyses (TGA) were performed on E. citriodora as well as on PMD, DEET and on the new mixture reaction to determine the weight-loss of selected samples at 33 °C (skin temperature) versus time. These records provide information on the evaporation rate of these different samples, which were compared with their repellent action on mosquitoes.
2 Materials and methods
2.1 Synthesis and analysis
2.1.1 Chemicals
E. citriodora essential oil was purchased from Albert-Vieille (F-Vallauris). Extracted by hydro-distillation in China, this oil was obtained mainly from the leaves of the tree. Table 1 sums up the list of different compounds present in this oil (data given from the manufacturer). 2-hydroxypropane-1,2,3-tricarboxylic acid monohydrate (citric acid – 99.5%), was used as a catalyst for the Prins reaction and dissolved in distilled H2O to obtain solutions at different concentrations. Na2SO4 (99%) was used as a drying agent, N,N-dimethylmethanamide (99.8%) as a solvent to analyse the mixture reaction by gas chromatography. iPrOH was used as a solvent for the modified oil, the pure oil and the PMD to test their repellent action in cage tests. All these reagents and solvents were supplied from Merck (D-Hohenbrunn). Pure PMD (99%) was obtained from Takasago (F-Paris) with a specific ratio between the two isomers (+)-cis and (-)-trans (62:38). At last, DEET was purchased from Sigma-Aldrich (D-Munich) with a purity of (95%).
Chemical composition of Eucalyptus Citriodora essential oil from Albert-Vieille (F-Vallauris).
| Component | % in weight |
| α-Thujene | 0.04 |
| α-Pinene | 2.10 |
| β-Pinene | 1.28 |
| Sabinene | 0.07 |
| Myrcene | 0.14 |
| α-Terpinene | 0.08 |
| Limonene | 0.17 |
| Cineol-1,8 | 0.49 |
| Cis-β-Ocimene | 0.09 |
| γ-Terpinene | 0.16 |
| p-Cymene | 0.07 |
| Terpinolene | 0.16 |
| Citronellal | 74.21 |
| Linalool | 0.22 |
| Neoisopulegol | 2.25 |
| Isopulegol | 4.68 |
| β-Caryophyllene | 1.13 |
| Terpinene-1-ol-4 | 0.16 |
| α-Terpineol | 0.09 |
| Citronellol | 5.95 |
| Citronellyl acetate | 1.33 |
| Nerol | 0.06 |
| Geranial | 0.03 |
| Geraniol | 0.1 |
| Sum of component | 95.06 |
2.1.2 Synthesis of para-Menthane-3,8-diol isomers
In a 50-ml round-bottomed flask 5.25–22 g of a 1–15 wt% citric acid aqueous solution and 3.7 g (24.0 mmol) of E. citriodora oil were charged and heated between 40 to 60 °C under agitation (450 tr/min). The biphasic medium was maintained at constant temperature between 6 to 15 h. Then, the organic phase, composed of PMD, monoterpenes, sesquiterpenes and others acetals were separated from the aqueous phase after several hours decantation and dried with sodium sulphate (Na2SO4).
2.1.3 GC analysis
GC analysis of the mixture medium were performed on a HP 6890 Series gas chromatograph fitted with a flame ionization detector (FID) and an electronic integrator. The capillary column used was a HP model 19091 J-413: HP-5 5% Phenyl Methyl Siloxane (30 m × 320 μm; film thickness 0.25 μm nominal). Helium was employed as a carrier gas at a flow rate of 104 ml/min and 65.6 kPa inlet pressure. Temperature program was: 50 °C at 10 °C/min, rising to 225 °C, and then remaining at 225 °C for a period of 15 min. Injector and detector were maintained respectively at 280 °C and 250 °C. Samples (1 μL) were injected neat with a 50:1 split ratio.
2.1.4 Identification and quantification method
All components were identified by the retention time of standard molecules from a specific GC program. The response factor of the internal standard solution was measured using GC with known amount of N,N-dimethylmethanamide and of reaction mixture.
2.1.5 Thermogravimetric analysis
Pure E. citriodora oil, PMD and the new mixture reaction samples of ∼ 13 mg were subjected to thermogravimetric analysis in a nitrogen atmosphere. A TGA7 from Perkin Elmer corp. (USA-Norwalk) was used. An isotherm-heating program was settled up at 33 °C (Human skin temperature on the forearm). In order to obtain a low-noise TG signal, a constant gas flow of 70 ml min−1 was set for all the tests. The precision of temperature measurement for the thermobalance is ± 1 °C. The continuous records of weight-loss and temperature were obtained and used to determine the evaporation rates (weight-loss % min−1) of the pure essential oil, PMD, DEET, (+)-citronellal and of the modified essential oil.
2.2 Mosquito repellent studies
2.2.1 Insects
Strain of A. aegypti mosquitoes from Bayer AG were reared according to the standard protocol at a temperature of 27 °C, a relative humidity of 60–80% and a 12:12 hour photoperiod. The light period (150 Lux) was set from 08:00 to 20:00. After hatching of the eggs, larvae were kept in H2O filled with a 1:1 mixture of tap- and deionised H2O, and fed with fishfood flakes (Tetra Min®). Before hatching, the pupae were transferred to a cage (40 × 30 × 20 cm) and provided with sugar solution (10% dextrose). Mosquitoes at an age of 9–14 days were used for the tests.
2.2.2 Volunteers
Five human volunteers aged between 20 and 26 participated in the mosquito cage test bioassay. No abnormal allergic reaction after application of the formulations was observed.
2.2.3 Laboratory tests
Human skin tests were conducted as described below.
2.2.3.1 Application of repellents
The skin of the forearm between wrist and elbow was used as a test area. Prior to the experiment, the skin was washed with fragrance-free soap and 50% iPrOH and then dried with a paper towel. An area larger than the test window was marked on the skin with a metal template. According to the United States EPA, 1 g of pump spray is applied per 600 cm2 skin. The marked area had a size of 98 cm2; therefore, 0.17 g were applied to the test area. The test substance was applied using a pipette with disposable tips, for each test person a new tip was used. The test substance was spread evenly on the skin of the forearm by hand wearing a latex glove.
2.2.3.2 Exposure to mosquitoes
Thirty mosquitoes were placed in a test cage that was fitted with a test window in the floor of the cage. The test window was closed by a metal slide and was opened by inserting a metal frame for the exposure of the treated quadratic (98 cm2) skin area (ventral) and untreated area (back) of the volunteer's forearms. This method was designed so that each volunteer served as his own control.
2.2.3.3 Zero control
Zero control is the untreated back of the forearm of the test person. A window frame with mosquito net is used to keep probing mosquito from successfully taking a blood meal. Biting pressure must exceed 10 probings in 30 s. After 30 s and more than 10 probings the mosquitoes were considered as active and suitable for the experiment.
2.2.3.4 Test proper
Each test person was assigned to one test cage. Between two tests a special air ventilation system was attached to the cage in order to prevent any accumulation of odours and active substances in the cage. The treated skin was exposed to the mosquitoes for a testing time of 2 min. In this time, the number of landings and bitings on the treated skin was noted and compared to the untreated control skin.
2.2.3.5 Determination of duration of protection
As far as there is no official protocol in EU, tests were based on the EPA draft guideline OPPTS 810.3700 (EPA, 2000). The criteria to define a complete protection time were dependent on the zero control. A 15 min margin of error was attributed on every result.
2.2.4 Statistical analysis
Data collected during evaluation of duration-of-protection tests were subjected to an analysis of variance (ANOVA) and t-test (P < 0.05) using the software SPPS (version 12.0 for windows). This statistical analysis was used to check the reliability of the results obtained from the bioassay and to verify if there are any significant differences in the documented protection times of the tested solutions.
3 Results and discussion
3.1 Transformation of Eucalyptus citriodora oil and synthesis of para-Menthane-3,8-diol isomers
The preparation of PMD isomers from (+)-citronellal contained in the E. citriodora oil involves the presence of an acidic medium as shown on Scheme 1. Citric acid was considered due to its natural production source [21] for the Prins reaction as a catalyst for the cyclisation of (+)-citronellal, which lead to a more natural way for the PMD synthesis.
H2SO4 was already used in the past to produce PMD from pure (+)-citronellal [22]. The present work shows the production of the same active through a similar reaction but using as a starting material, E. citriodora. This oil contains more than 24 compounds mainly monoterpenes and sesquiterpenes and the majority may react in such acidic medium and might involve the apparition of other entities (side products). In this study, different parameters were investigated during the cyclisation reaction: the acid concentration, the temperature of the medium, the reaction time and the quantity of the aqueous solution. All these parameters were optimized and contributed to first improve the conversion of citronellal and then reach the highest selectivity of PMD during the reaction. The results are shown in Table 2.
Concentration of citric acid, temperature, reaction time and quantity of water in the medium, are parameters considered for the cyclisation of (+)-citronellal. Conversion and selectivity were calculated from appropriate programs.
| Run | Concentration Acid (%) | Temperature (°C) | Time (h) | Water wieght (g) | Conversion of (+)-citronellal (%) | Selectivity of PMD (%) | Ratio (+)-cis/(−)-trans |
| 1 | 1 | 50 | 15 | 5.25 | 46.3 | 90.3 | 68/32 |
| 2 | 5 | 50 | 15 | 5.25 | 81.2 | 66.7 | 71/29 |
| 3 | 7 | 50 | 15 | 5.25 | 82.1 | 79.6 | 65/35 |
| 4 | 10 | 50 | 15 | 5.25 | 83.7 | 78.9 | 68/32 |
| 5 | 15 | 50 | 15 | 5.25 | 77.7 | 76.9 | 66/34 |
| 6 | 7 | 50 | 6 | 5.25 | 55.1 | 85.7 | 68/32 |
| 7 | 7 | 50 | 9 | 5.25 | 67.2 | 82.8 | 66/34 |
| 8 | 7 | 50 | 15 | 10.5 | 85.3 | 78.2 | 66/34 |
| 9 | 7 | 50 | 15 | 17.5 | 82.8 | 79.2 | 65/35 |
| 10 | 7 | 50 | 15 | 22 | 84.8 | 80.4 | 66/34 |
Table 2 shows clearly the conversion of (+)-citronellal as function of different parameters. Run no. 3 led to the best production of PMDs with 82% conversion of citronellal and 80% selectivity of PMD. Run no. 9 provided similar results but it needed three times more acidic medium to reach the same selectivity. It was necessary to stir in a 50 ̊C bath for 15 h, which is conceivable for a mass production perspective. The synthesis is perfectly reproducible even if the stirring action is highly determinant to provide the best conversion. The modified oil was subjected to GC analysis using a method of choice and then calculations were done to determine the composition of the reaction mixture. An example is shown on Fig. 1 with the production of PMD using a 7% of citric acid solution for 15 h at 50° (run no. 3).
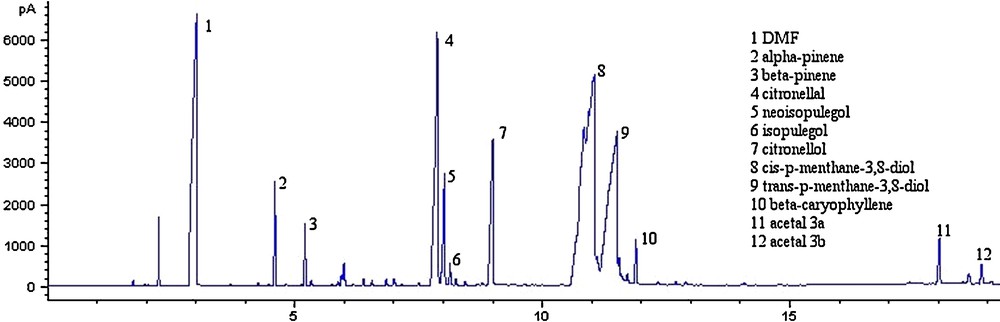
GC chromatogram of the synthesis of para-Menthane-3,8-diol from Eucalyptus citriodora oil. Reaction using 7% of citric acid at 250 °C for 15 h (run n°3). GC parameters are described in the experimental section.
Peaks 8 and 9 correspond respectively to the (+)-cis and (-)-trans PMDs. (-)-cis and (+)-trans are also present in the reaction mixture at a very low concentration. Around 13% of (+)-citronellal remained in the solution, which match a conversion of 82%. The selectivity for the PMD production reached 80% with 64% concentration in the final mixture ((+)-cis/(-)-trans: 65:35) on run no. 3. Citronellol (peak 7) remained in the modified oil at 5.95% as well as iso/neosipulegol (peaks 5/6) with respectively 4.7% and 2.2% of the total mixture. Additionally, PMD acetals 3a and 3b (Scheme 2) were produced at a very low concentration, respectively 1.40% and 0.35%, as already found in the literature [23]. Several syntheses were done using other parameters showing a large concentration of these acetals at the end, which is not the case on run no. 3. There is no evidence that these acetals may provide any repellent action on mosquitoes.
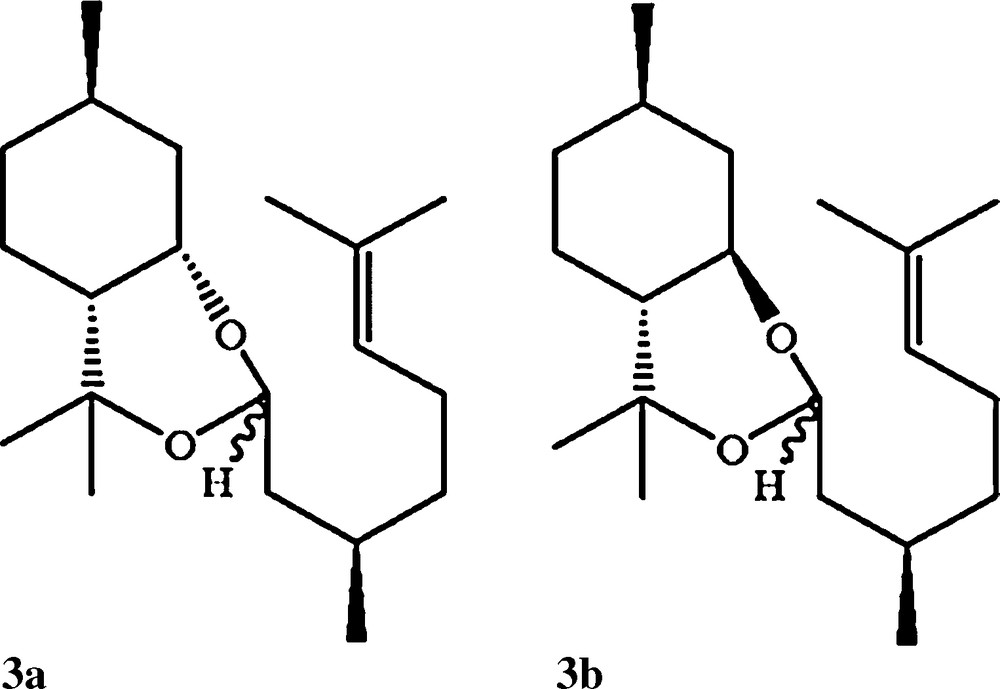
Structure of two PMD acetals produced during the cyclisation of (+)-citronellal (peaks 11/12 on Fig. 1).
As shown on Fig. 1, many monoterpenes and sesquiterpenes remain in the modified oil. The authors assume that their concentrations stay constant in such an acidic medium compared to the original oil. The modified oil was not subjected to any separation process and was then tested with this composition on a bioassay to assess its repellent action on A. aegypti mosquitoes.
3.2 Mosquito repellent study
This study evaluated the repellence of the new reaction mixture by using cage tests on a bioassay with A. aegypti mosquitoes. The screening was set up following the method previously described (Experimental part). On two days test, Human volunteers applied five solutions on their forearms: a 20% of pure E. citriodora oil, 13% and 20% of pure PMD as both standard control, a 20% DEET and a 20% solution of the new modified oil. The new mixture was obtained from the process previously described as no. 3 in Table 2. Fig. 2 exhibits results of the different samples used in the cages tests showing great differences on the time protection.
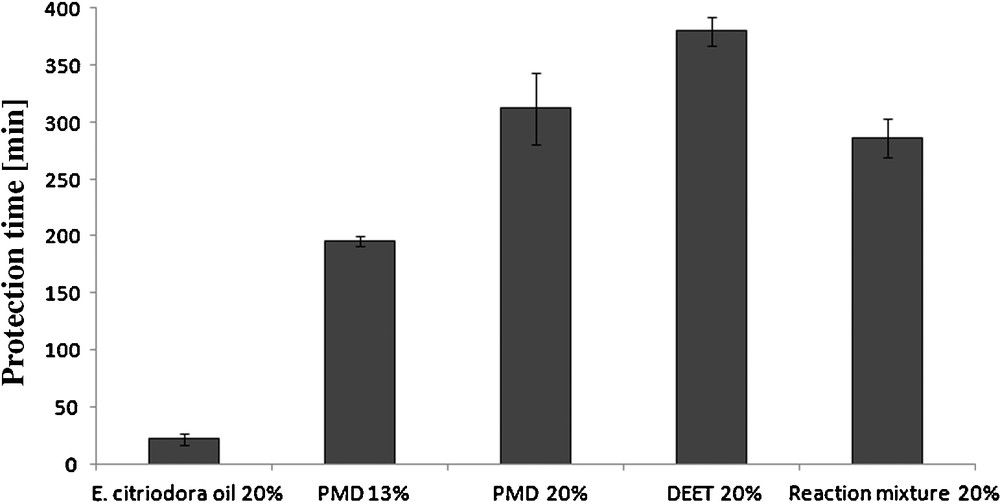
Different protection time values on human volunteers with Aedes aegypti of solutions previously described.
As a result, the pure essential oil solution has shown a short repellent activity on A. aegypti with a mean protection time of 22 min, which is less than previous figures found in the literature. E. citriodora has been credited with anti-inflammatory, antibacterial, and antifungal activity, and, recently has gained popularity as an insect repellent [24]. In the present study, this oil has never proved a great repellent action at any specific concentration (unpublished data from Drapeau et al.) despite all the data found in the literature. It is mainly employed in commercial products as a secondary active in addition to other actives. Besides, thermogravimetric analysis will show further in this paper its high evaporation rate, which may affect its lasting protection.
Hundred and ninety-six minutes was reached with the pure PMD solution at 13% concentration and 312 min for the 20%, which corresponds to the mean values of PMD protection time at these concentrations as shown on previous reports [25]. PMD has a faint mint odour and is slightly soluble in water.
DEET was also tested on a bioassay since this component is used in many commercial products all over the world. The 20% concentrated solution provided 380 min complete protection against A. aegypti mosquitoes using the same mosquito strain and protocol. In the literature, PMD preparations appear to provide a lower protection time than DEET (up to 1.5 lower) and require more frequent reapplication to maintain the same level of protection [26].
In comparison to pure E. citriodora oil, the modified oil caused a great increase on the time protection on human volunteers against A. aegypti with a mean value of 303 min.
ANOVAs conducted on the protection time of these five samples revealed no significant differences between the modified oil, the DEET and PMD solutions at 20% concentrated. On the contrary, statistical calculations showed a significant difference between the new reaction mixture (PMD 13% + mono/sesquiterpenes + acetals) and the 13% pure PMD solution from Takasago with an improvement up to 50% on the protection time.
The modified oil is mainly composed of PMD cis/trans isomers (64%), (+)-citronellal (13%), citronellol (6%) and many others terpenes in lower concentrations. However, the presence of these side products and residues do not affect the repellency of PMD and even increases drastically its action. Unpublished data show that individually, these molecules in alcoholic solutions and using the same protocol, present no efficient protection over time against A. aegypti mosquito at such low concentration. Besides, investigations were carried out with combinations of PMD/citronellal and PMD/citronellol with the same concentrations, as they are present in the modified oil. The results indicate no significant differences between these two combinations and pure PMD solutions.
There is no obvious explanation on the high protection action of the modified oil compare to the pure PMD except that the side components may act in synergism all together to provide a certain protection even if they did not show any repellent properties separately.
3.3 Thermogravimetric analysis
TGA thermograms for DEET, PMD, E. citriodora, E. citriodora modified and (+)-citronellal are given on Fig. 3. From the thermogram, it is found that DEET and PMD undergo the same loss of weight over time at 33 °C to reach about 2% loss after 24 h. This behavior is directly related to the vapor pressure of the two molecules, which is comparable. PMD and DEET have both a low vapour pressure inducing a weak volatility (0.00109/0.0056 mm Hg at 25 °C) vapor pressure calculated using Advanced Chemistry Development (ACD/Labs) Software V8.14 for Solaris (Ó 1994–2008 ACD/Labs). Despite their similar volatility over time, these two molecules display a different behaviour on the mosquitoes as shown on Figs. 2 and 3. This difference is not significant. The lost of activity is certainly due to the penetration of these two components through the skin. The PMD molecule is much smaller than the DEET and may cross easily the lipid layers of the skin. On another hand, the hydroxide groups of PMD might interact with the polar heads of the lipids and involve a highest retention of the PMD molecules on the upper skin layers as for the DEET. Also the receptors’ acuity on the mosquito's antennas might be a parameter to explain the difference of behaviour between these two molecules.
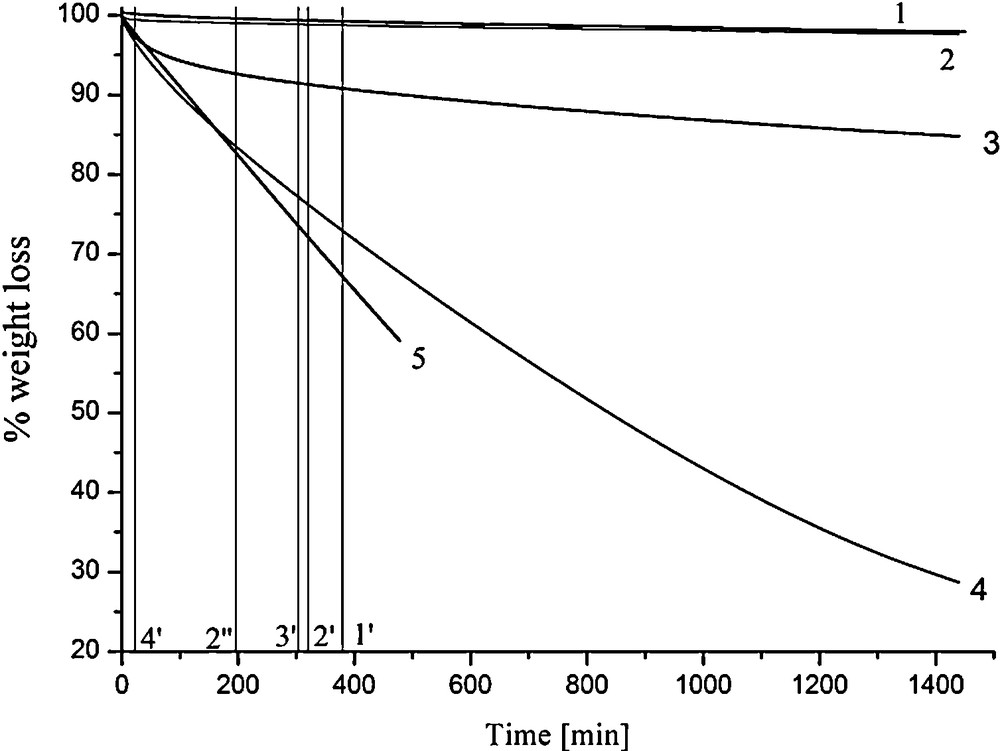
TGA curves of the evaporation rate of DEET (1), PMD (2), modified Eucalyptus citriodora oil (3), E. citriodora oil (4) and pure (+)-citronellal (5) under a nitrogen atmosphere at 33 °C ± 1 °C. The vertical lines represent the protection time obtained from the repellent study for the DEET solution (1′), the PMD solution at 20 wt% and 13 wt% (2′-2′’), the modified oil (3′) and the pure E. citriodora oil (4′).
TGA of the E. citriodora essential oil exhibits a completely different curve. After 24 h, 70% of the oil evaporated. Essential oils usually have relatively high vapor pressures inducing high volatilities over time. Hydrocarbonated monoterpenes are very well known to display a high evaporation. The 6% terpenes present in the E. citriodora oil may evaporate first and should be responsible for the weak protection against the mosquitoes (around 25 min). The citronellal curve shows a linear degradation over time, which is significant over a long period. Repellent study (not presented) on this molecule did not show any repellent action on A. aegypti mosquito in contrast to others studies found in the literature.
Finally, the modified oil was subjected to a TGA analysis. After 24 h at 33 °C, the reaction mixture lost 15% of its weight showing a very slow degradation. This oil is composed of around 64% PMD, 13% citronellal and the rest of hydrocarbonated monoterpenes or sesquiterpenes and acetals. The first losses are induced by the terpenes as previously mentioned. The thermogram presents a plateau reached after few hours as also found with the PMD and DEET analysis. The presence of PMD in the reaction mixture and its low degradation logically displays a good protection time on the mosquitoes at around 300 min for a 20% in an alcoholic solution. However, the presence of 13% of citronellal in the solution should lead to a greater deformation of the curve, which is not the case. The authors recall that PMD may self-associate in hydro-alcoholic solutions and form aggregates, which could slow down the skin rate penetration and then provide a longer repellency action.
4 Conclusion
PMDs were synthesized from E. citriodora oil by using an easy, fast, green and cost effective process. With adapted choices of acid, temperature and time reaction, cyclisation of (+)-citronellal from the oil induces the production of cis and trans PMDs with a high selectivity. In a bioassay, this natural PMD in combination with others acetals and terpenes gave a long lasting protection against A. aegypti mosquitoes. Three hundred and three minutes of complete protection on human beings was reached with the new mixture (20% in iPrOH). A comparison with the reference active DEET and the modified oil showed no significant difference in the protection time between both actives at the same concentration.
Acknowledgements
The authors are grateful to the “Bayerische Forschungsstiftung” for generous funding as well as the volunteers for the repellent study.


