1 Introduction
Heterocyclic compounds bearing thiazolidineones have long been the focus of synthetic chemistry due to their broad spectrum of applications in biological, pharmaceutical, and material area [1,2]. Specifically, thiazolidines containing a ketonic group and an alkyl group have attracted much attention due to their interesting and unique properties such protein–nucleic acid interactions, antiviral activity and antidiabetic activity. Structural activity relationship (SAR) studies of the thiazolidine derivatives showed that the substituent groups on the rings are all critical for the activity. However, to our knowledge, only a few methods were developed to construct polysubstituted thiazolones. Moreover, some of these protocols have not been entirely satisfactory because of such drawbacks as low yields, long reaction time and cumbersome experimental processes [3,4].
Multicomponent coupling reactions are emerging as useful tools for synthesizing small drug-like molecules with several degrees of structural diversity [5–10]. Pioneering work by several research groups in this area has already established the versatility and uniqueness of one-pot multicomponent coupling protocols as a powerful methodology for the synthesis of diverse structure scaffolds required in the search of novel therapeutic molecules [11–19].
Presence of these moieties in organic molecules imparts them with the extensive range of biological and pharmacological properties, such as anti-diabetic, anti-cancerous and antimicrobial activities. Moreover, these compounds are also useful as conducting materials. Many of the methods reported for the synthesis of organic compounds are associated with the use of hazardous organic solvents, long reaction time, and lack of general applicability. Thus, we developed a simple one-pot synthesis of novel 9-phenyl-3,9-dihydro-chromeno[2,3-d]thiazol-2-ones derivatives under catalyst free condition.
2 Result and discussion
Our approach could comprise the relay process of the following sequences (Scheme 1):
- 1. two component addition reaction;
- 2. two component phenol-alcohol dehydration cyclization process.
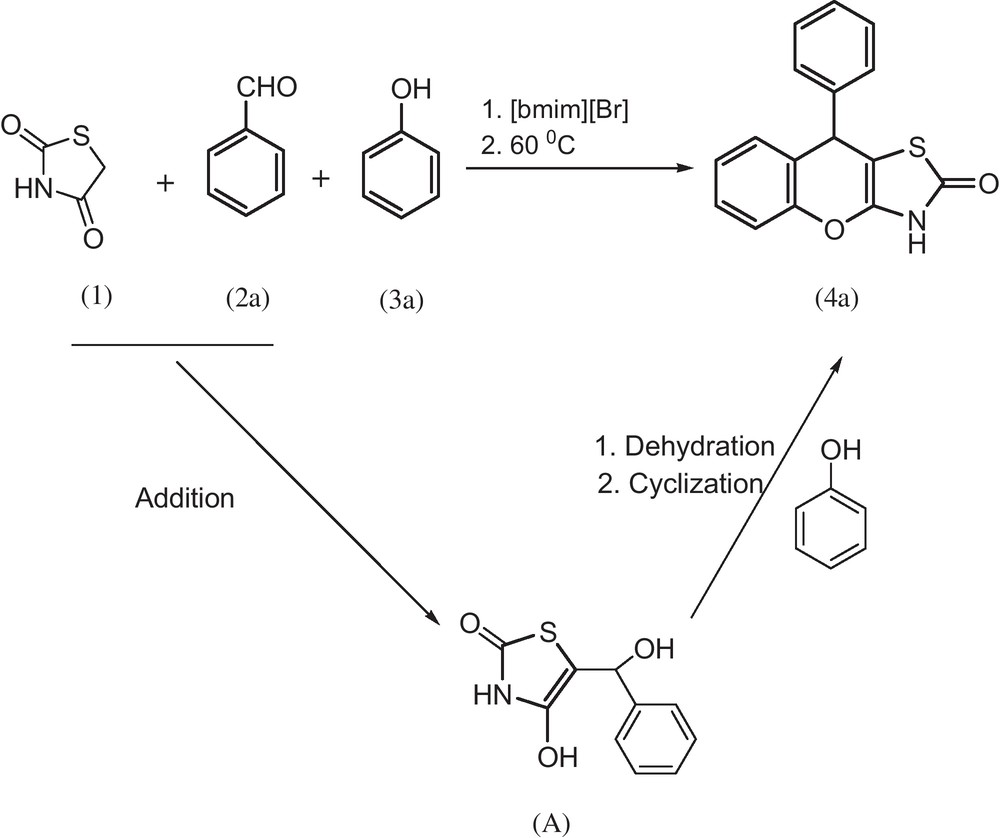
Synthesis of 9-Phenyl-3,9-dihydro-chromeno[2,3-d]thiazol-2-one.
Thiazolidine-2,4-dione (1) reacts with benzaldehyde (2a) and phenol (3a) to afford the product 9-Phenyl-3,9-dihydro-chromeno[2,3-d]thiazol-2-one (4a) in excellent yield (Scheme 1, 89%). The reaction of thiazolidine-2,4-dione (1) with benzaldehyde (2a) could rapidly form (A). Then (A) reacts with phenol (3a) and promotes the alcohol-phenol dehydration-cyclization and coupling processes to afford the target compound (4a). To our knowledge, the core of two reactions catalyzed by acidic character of phenol, namely addition reaction and dehydration cyclization with coupling reaction, has not been reported previously.
Screening of the reaction conditions was established suitable solvents, the mole ratio of reactants as well as temperature for the desired MCRs (Table 1).
Optimization of reaction conditions (solvent and mole ratio of the reactants) for the catalyst free multicomponent reactions.a
| S. No. | Solvent | Mole ratio (1:2a:3a) | t (h) | Temp. (°C) | Yield (%) |
| 1 | Dioxane | 1:1:1 | 7 | 35 | 75 |
| 2 | CH3CN | 1:1:1 | 7 | 35 | 72 |
| 3 | DMF | 1:1:1 | 10 | 35 | 52 |
| 4 | Toluene | 1:1:1 | 5.2 | 35 | 70 |
| 5 | None | 1:1:1 | 8 | 35 | Complex |
| 6 | DMSO | 1:1:1 | 10 | 35 | 50 |
| 7 | THF | 1:1:1 | 7 | 35 | 60 |
| 8 | [NEt3][Ac] | 1:1:1 | 6 | 35 | 78 |
| 9 | [bmim][Cl] | 1:1:1 | 5.5 | 35 | 75 |
| 10 | [bmim][Br] | 1:1:1 | 4 | 35 | 78 |
| 11 | [bmim][Br] | 1:1:1.1 | 4 | 35 | 80 |
| 12 | [bmim][Br] | 1:1:1.2 | 4 | 35 | 88 |
| 13 | [bmim][Br] | 1:1:1.3 | 4 | 35 | 76 |
| 14 | [bmim][Br] | 1:1:0.9 | 4 | 35 | 72 |
| 15 | [bmim][Br] | 1:1:0.8 | 4 | 35 | 70 |
| 16 | [bmim][Br] | 1:1:1.2 | 4 | 50 | 90 |
| 17 | [bmim][Br] | 1:1:1.2 | 4 | 60 | 95 |
| 18 | [bmim][Br] | 1:1:1.2 | 4 | 90 | 92 |
a The reaction were carried out amongst thiazolidine-2,4-dione (1), aromatic aldehyde (2a) and phenol (3) in a solvent (10 mL).
It was exciting that the chosen solvents such as dioxane, N,N–dimethylformamide (DMF), acetonitrile (CH3CN), dimethylsulfoxide (DMSO), toluene, etc. were suitable for the MCRs (Table 1, entries 1–10). The ionic liquid (bmimBr) proved to be the best among them (Table 1, entry 10) while under solvent free conditions, a complex result was obtained (Table 1, entry 5). To modulate the ratio of reactants and improve the yield, we examined various ratios of thiazolidine-2, 4-dione (1), benzaldehyde (2a) and phenol (3a) by using acetonitrile as a solvent Table 1, entries 11–15). The best result is obtained when the ratio of thiazolidine-2, 4-diones (1), benzaldehyde (2a) and phenol (3a) is 1:1:1.2 to afford the product 4a, i.e. Entry 12. Further, optimization of temperature was done (Table 1, entries 16–18) and we found the best yield was obtained at 60 °C (entry 17).
With the optimized condition in hand, we examine the scope of the multicomponent reaction (Table 2, entries 1–13). We were pleased to find that the reaction proceeded smoothly, and the desired products were afforded in excellent yields. Meaningfully, the substituted group on the thiazolidine ring could not be selectively induced by changing the addition order of the aromatic aldehyde and phenol under the same employed conditions. The ionic liquid used for the transformation (cylization followed by dehydration) was recovered and used for the further reaction and gives good yield for the further reactions as shown in Table 3.
Catalyst free one-pot synthesis of 9-Phenyl-3,9-dihydro-chromeno [2,3-d] thiazol-2-one via MCRs between thiazolidine-2,4-dione, aromatic aldehyde, phenols (10 mmol).
| S. No. | Reactant2 | Reactant3 | Product4 | t (h) | Yield (%) |
| 1 | 4 | 95 | |||
| 2 | 4.5 | 90 | |||
| 3 | 4.5 | 92 | |||
| 4 | 4 | 92 | |||
| 5 | 4 | 92 | |||
| 6 | 3.5 | 90 | |||
| 7 | 4.5 | 94 | |||
| 8 | 5 | 96 | |||
| 9 | 5 | 88 | |||
| 10 | 5.5 | 91 | |||
| 11 | 6 | 90 | |||
| 12 | 5.5 | 87 | |||
| 13 | 5.5 | 89 |
Optimization of the activity of ionic liquid after reuse.
| S. No. | No. of cycle | Yield (%) |
| 1 | I | 95 |
| 3 | II | 94 |
| 3 | III | 94 |
| 4 | IV | 92 |
3 Experimental
In a round bottom flask (100 mL), a mixture of thiazolidine-2,4-dione (10 mmol), benzaldehyde (10 mmol) and phenol (10 mmol) in ionic liquid [bmimBr] (20 mL) was taken and further heated at 60 °C for appropriate time. The completion of the reaction was monitored by thin layer chromatography (ethyl acetate: n-hexane: 20:80). After the completion of reactions, the compound was isolated from the reaction mixture by the addition of ethyl ether (three times × 30 mL) and further washed with brine solution (three times × 30 mL). Then ethyl ether was evaporated under reduced pressure to afford the corresponding product. Similarly, the corresponding products (Table 2, entries 2–13) were synthesized using the above methodology.
4 Conclusion
In conclusion, a series of biologically and pharmacologically active 9-Phenyl-3,9-dihydro-chromeno[2,3-d]thiazol-2-one derivatives have been synthesized via one-pot three component condensation of an aldehyde, phenol, and thiazolidine-2,4-dione in excellent yields within a practical reaction time. The advantages offered by an ionic liquid as a solvent versus known organic solvents are: (i) the ionic liquid has high vapor pressure; (ii) highly stable; (iii) reusable; and (iv) environmentally benign. The exploration of ionic liquid for other multicomponent reactions leading to biologically active compounds is underway.
Analytical data of the product 1: IR (ν in cm−1) 3126.05, 3042.01, 2825.24, 1738.29, 1680.53, 1391.01, 1343.18, 1231.66, 1164.92, 891.09, 807.37, 717.38, 617.91, 512.63, 405.59; 1H NMR (300 MHz, d-DMSO) δ 12.086 (s, 1H), 7.497-7350 (d-9H), δ 4.365 (s, 1H); 13C NMR (300 MHz, d-DMSO) δ for carbonyl carbon (180.79), δ for aromatic carbon (124.76, 124.05, 123.52, 122.65, 122.32, 122.03, 121.89, 121.34, 121.09, 119.52, 119.12, 118.76), δ for alkenic carbon (116.52, 113.89), benzylic carbon (50.46).
Acknowledgement
We gratefully acknowledge financial support from University Grant Commission (UGC), India.


