1 Introduction
The development of highly efficient and sustainable procedures has become a major driving force to assemble the biologically active compounds in the field of academia and industry [1]. A multicomponent reaction (MCR) is a process in which three or more components are combined together in a single reaction vessel to produce a final product [2]. The versatility and effectiveness as potential multicomponent substrate has been used in various MCRs such as Hantzsch reaction [3], Biginelli reaction [4], Schopfs tropinone synthesis [5], Tietze's reaction [6], Ugi [7] and Mannich type reactions [8].
Nevertheless, the development of MCRs is still in demand. In this context, ortho-quinone methides (O-QMS) intermediate were utilized in many tandem processes [9] and [4+2] cycloaddition with a wide range of dienophiles [10]. However, with carbon nucleophiles, only limited works have been reported [11]. It is difficult to create the reaction conditions for proper exploitation of O-QMs in reactions with carbon nucleophiles for organic synthesis [9].
Xanthene and its derivatives are known as an important class of heterocyclic compounds, widely used as lecodyes [12], pH-sensitive fluorescent materials for visualization of biomolecules [13] and are utilized in laser technologies due to their photochemical and photophysical properties. They possess diverse biological and therapeutic properties such as anti-inflammatory [14], antiviral [15] and antibacterial activities [16]. These compounds are being utilized as antagonists for paralyzing action of zoxazolamine [17] and in photodynamic therapy [18]. Among the molecules of this class, benzoxanthen is a prominent structural motif found in various natural products [19] and synthetic compounds with important biological activities [20].
Several methods have been used for the synthesis of tetrahydrobenzo[a]-xanthen-11-one and benzo[f]chromen-3-one derivatives [21]. These procedures have limitations of long reaction time, harsh reaction conditions and often required expensive catalysts. As a consequence, the development of a mild and practical procedure for accessing these benzoxanthen derivatives remains an elusive goal. Recently, lanthanide compounds have become attractive candidates for use as Lewis acid catalysts in organic chemistry with numerous applications as promoters [22]. The introduction of electronegative ligands such as chloride enhances the activity by increasing the Lewis acidity of the metal [23]. These catalysts offer several advantages including mild reaction conditions, cleaner reactions, shorter reaction times, lower catalyst loading and simple experimental procedures with high yields.
In this context, cerium(III) chloride has been received as an inexpensive, non-toxic, water tolerant and potentially useful Lewis acid catalyst [24] for several organic reactions [25]. The activity of cerium(III) chloride was increased in combination with NaI [26]. As a part of our ongoing program on the development of new synthetic methods [27] and to examine the behaviour of cerium(III) chloride as catalyst [28] in the synthesis of organic compounds [29] herein, we wish to report a mild and efficient protocol for the synthesis of tetrahydrobenzo[a]-xanthen-11-one derivatives via one-pot, three-component reaction of aldehydes, β-naphthol and cyclic 1,3-dicarbonyl compounds using cerium(III) chloride as catalyst in methanol at 50 °C (Scheme 1).
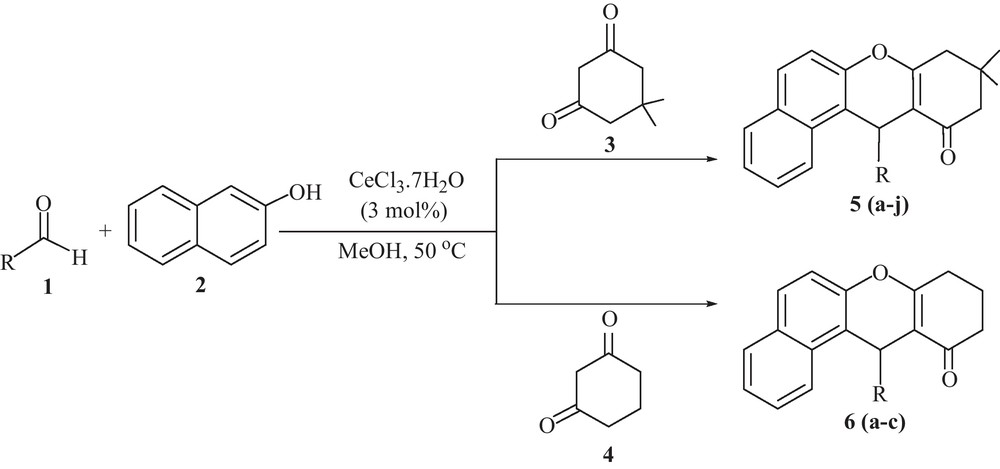
CeCl3.7H2O catalyzed condensation of β-naphthol, aldehydes and cyclic 1,3-dicarbonyl compounds.
2 Results and discussion
To find the optimum reaction conditions, a mixture of β-naphthol (1 mmol), benzaldehyde (1 mmol) and 5,5-dimethyl-1,3-cyclohexanedione (1.2 mmol) in methanol (5 mL) were stirred at 50 °C under various reaction conditions (Table 1). The reaction was completed within 8 h in the absence of catalyst. However, under the same reaction conditions, by employing 1 mol% of CeCl3.7H2O, the reaction afforded expected product up to 25% yield within 5 h of reaction time (entry 2, Table 1).
Optimization of the concentration of CeCl3.7H2O for the synthesis of tetrahydrobenzo[a]-xanthen-11-ones.a
| Entry | CeCl3.7H2O (mol%) | Time (h) | Yield (%)b |
| 1 | 0 | 8 | 10 |
| 2 | 1 | 5 | 25 |
| 3 | 2 | 2 | 60 |
| 4 | 3 | 2 | 90 |
a Reaction conditions: benzaldehyde 1 (1.0 mmol), β-naphthol 2 (1.0 mmol), 5,5-dimethyl-1,3-cyclohexanedione 3 (1.2 mmol), catalyst CeCl3.7H2O, solvent CH3OH (5 mL), temp. 50 °C.
b Isolated yields.
With this optimistic result in hand, we further investigated the best reaction conditions by using different amounts of CeCl3.7H2O. An increase in the quantity of CeCl3.7H2O from 1 to 3 mol% not only decreases the reaction time from 5 to 2 h but also increased the product yield from 25 to 90% (entry 4, Table 1). In a search for an even higher yield, we decided to employ NaI as an additive with CeCl3.7H2O (CeCl3.7H2O-NaI system). In this context, the effect of the amounts of the NaI and CeCl3.7H2O was evaluated. When the reaction was carried out by using CeCl3.7H2O-NaI (3 mol%) in methanol (5 mL), the product yield was nearly the same (entry 3, Table 2) as the yield obtained with CeCl3.7H2O (3 mol%) (entry 2, Table 2). The rate of this reaction was slightly improved by adding 5 mol% of CeCl3.7H2O-NaI, and the desired product was obtained in 2 h. Thus we found that 3 mol% CeCl3.7H2O is better suited than the CeCl3.7H2O-NaI system for the optimum yield of tetrahydrobenzo[a]-xanthen-11-one derivatives (Table 2).
Evaluation of catalytic activity of various catalysts for the synthesis of tetrahydrobenzo[a]-xanthen-11-ones.a
| Entry | Catalyst | Mol % | Time (h) | Yield (%)b |
| 1 | CuSO4 | 10 | 8 | 25 |
| 2 | CeCl3.7H2O | 3 | 2 | 90 |
| 3 | CeCl3.7H2O-NaI | 3 | 2 | 91 |
| 4 | CeCl3.7H2O-NaI | 5 | 2 | 93 |
| 5 | Y(OTf)3 | 10 | 5 | 35 |
| 6 | AlCl3 | 10 | 5 | 45 |
| 7 | ZnCl2 | 10 | 5 | 30 |
| 8 | Sr(OTf)2 | 10 | 5 | 85 |
a Reaction conditions: Benzaldehyde 1 (1.0 mmol), β-naphthol 2 (1.0 mmol), 5,5-dimethyl-1,3-cyclohexanedione 3 (1.2 mmol), different catalysts, solvent CH3OH (5 mL), temp. 50 °C.
b Isolated yields.
Further, we have also scrutinized this reaction by using various Lewis acid catalysts such as CuSO4, CeCl3.7H2O, Y(OTf)3, AlCl3, ZnCl2, and Sr(OTf)2. Comparative studies show that CeCl3.7H2O is a more effective catalyst for this condensation reaction (entry 2, Table 2).
It is well known that the reaction medium plays an important role in the catalytic reaction. To study the influence of the nature of solvent for this reaction using CeCl3.7H2O as a catalyst was carried out at a temperature (50° C) in different solvents, such as acetonitrile, THF, toluene, methanol and dichloromethane (Table 3). The highest reaction activity was achieved in the system using methanol as a solvent in comparision to other solvents under similar reaction conditions (entry 4, Table 3). The results show that the catalytic performance is strongly affected by the type of solvent but a direct correlation between solvent properties and their efficiency could not be established in any case.
Solvent effect on reaction of β-naphthol, benzaldehyde and 5,5-dimethyl-1,3-cyclohexanedione.a
| Entry | Solvent | Yield (%)b |
| 1 | Acetonitrile | 42 |
| 2 | THF | 25 |
| 3 | Toluene | 12 |
| 4 | Methanol | 90 |
| 5 | Dichloromethane | 30 |
a Reaction conditions: Benzaldehyde 1 (1.0 mmol), β-naphthol 2 (1.0 mmol), 5,5-dimethyl-1,3-cyclohexanedione 3 (1.2 mmol) catalyst CeCl3.7H2O (3 mol%), different solvent (5 mL), time (2 h), temp. 50 °C.
b Isolated yields.
Encouraged by these remarkable results, we screened a variety of aromatic and heterocyclic aldehydes with β-naphthol and 5,5-dimethyl-1,3-cyclohexanedione to obtain the corresponding products. The results are summarized in Table 4. In all cases, various aromatic aldehydes with electron-donating and electron-withdrawing substituents were reacted successfully giving the products in good to excellent yields. It was observed that substituents in the aromatic ring of aldehydes have a delicate effect on the reaction process. Aromatic aldehydes with electron-withdrawing groups reacted faster than those with electron-donating groups. In order to broaden the scope of the present method, the replacement of 5,5-dimethyl-1,3-cyclohexanedione with 1,3-cyclohexanedione was also examined. Under similar conditions, the reactions proceeded steadily to afford a series of 12-phenyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one derivatives with good yields (Entries 11–13, Table 4).
Synthesis of tetrahydrobenzo[a]-xanthen-11-one derivatives using CeCl3.7H2O.a
| Entry | Aldehydes (R) | 1,3-dicarbonyl compounds | Product | Time (h) | Yield (%)b |
| 1 | C6H5 | 3 | 5a | 2 | 90 |
| 2 | 4-ClC6H4 | 3 | 5b | 2.5 | 95 |
| 3 | 4-BrC6H4 | 3 | 5c | 2.5 | 95 |
| 4 | 4-H3CC6H4 | 3 | 5d | 3 | 89 |
| 5 | 4-CH3OC6H4 | 3 | 5e | 3.5 | 87 |
| 6 | 4-O2NC6H4 | 3 | 5f | 2 | 90 |
| 7 | 4-HOC6H4 | 3 | 5g | 2.5 | 83 |
| 8 | 2-Naphthyl | 3 | 5h | 2 | 89 |
| 9 | Piperonyl | 3 | 5i | 2 | 76 |
| 10 | 2-Thienyl | 3 | 5j | 2 | 78 |
| 11 | C6H5 | 4 | 6a | 3 | 87 |
| 12 | 4-ClC6H4 | 4 | 6b | 2.5 | 94 |
| 13 | 4-O2NC6H4 | 4 | 6c | 2 | 91 |
a Reaction conditions: Benzaldehyde 1 (1.0 mmol), β-naphthol 2 (1.0 mmol), 1, 3-dicarbonyl compounds 3 & 4 (1.2 mmol), catalyst CeCl3.7H2O (3 mol%), solvent CH3OH (5 mL), temp. 50 °C.
b Isolated yields.
A tentative mechanism for the formation of tetrahydrobenzo[a]xanthen-11-ones 5 (a-j) is proposed in Scheme 2. By following the literature [30], we suppose that the reaction might have proceeded via ortho-quinone methides intermediate (9), which was formed by the nucleophilic addition of β-naphthol (2) to aldehydes (1) catalyzed by CeCl3. Subsequent substitution of the oxygen atom, coordinated by CeCl3 with 5,5-dimethyl-1,3-cyclohexanedione (3), afforded 11 and 12. The compound 12 eliminated one molecule of H2O and afforded desired product 5 (a-j).
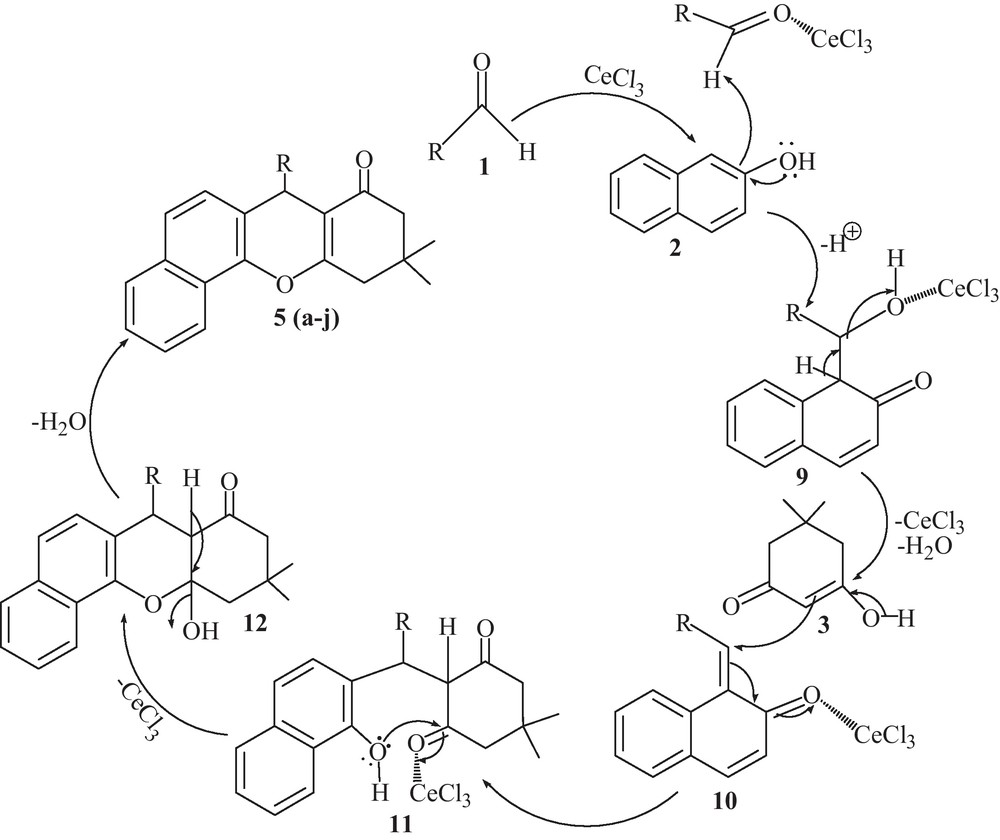
Proposed mechanism for the condensation reaction of aldehydes, β-naphthol and 5,5-dimethyl-1,3-cyclohexanedione.
Further, to realize the generality and versatility of the catalyst, this novel protocol was extended for the condensation of aromatic aldehydes, β-naphthol and Meldrum's acid. During the experiments, it was observed that different aromatic aldehydes bearing electron-withdrawing groups reacted smoothly in the presence of 3 mol% of CeCl3.7H2O at 50 °C in methanol to afford the high yield of benzo[f]chromen-3-ones (Scheme 3, Entries 2 and 3, Table 5).
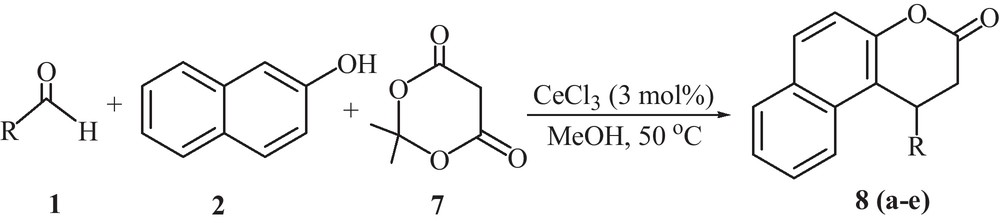
Synthesis of benzo[f]chromen-3-one derivatives using Meldrum's acid.
Synthesis of benzo[f]chromen-3-one derivatives catalyzed by CeCl3.7H2O.a
| Entry | R | Product | Time (h) | Yield (%)b |
| 1 | C6H5 | 8a | 3.5 | 89 |
| 2 | 4-ClC6H4 | 8b | 2 | 92 |
| 3 | 4-FC6H4 | 8c | 2.5 | 94 |
| 4 | 4-CH3OC6H4 | 8d | 6 | 70 |
| 5 | 2-Thienyl | 8e | 3 | 80 |
a Reaction conditions: Aldehydes 1 (1.0 mmol), β-naphthol 2 (1.0mmol), Meldrum's Acid 7 (1.2 mmol), catalyst CeCl3.7H2O (3 mol%), solvent CH3OH (5 mL), temp. 50 °C.
b Isolated yields.
Mechanistically, to clarify Scheme 3 for the synthesis of benzo[f]chromen-3-one derivatives, a mechanism was proposed [31]. The reaction may be rationalized by the condensation of β-naphthol (2) with aldehydes (1) catalyzed by CeCl3 and the initial formation of ortho-quinone methides intermediate (9) takes place which may be attacked by enol tautomer of Meldrum's acid (7) leading to the intermediate 13. The intermediate 13 may undergo formation of benzochromen-3-one derivatives as product 8 (a-e) followed by the removal of acetone and carbon dioxide molecules (Scheme 4).
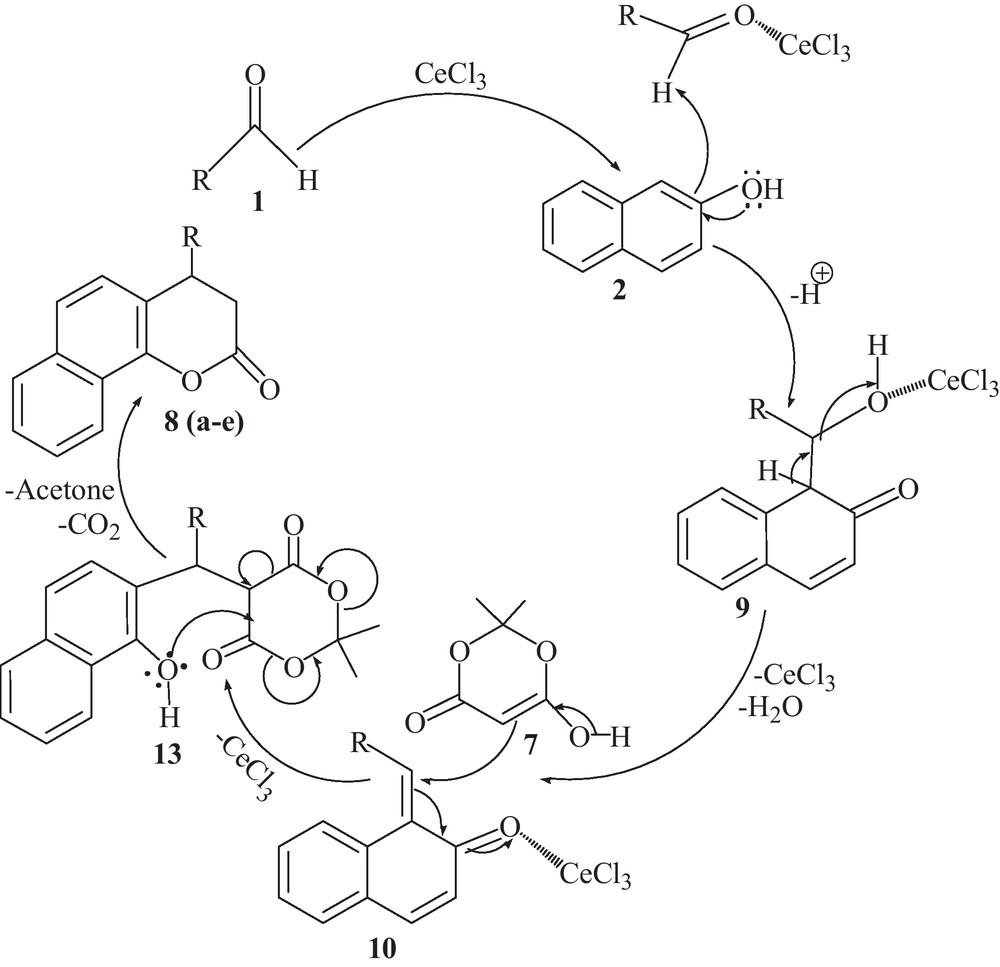
Proposed mechanism for the condensation reaction of aldehydes, β-naphthol and Meldrum's acid.
3 Conclusions
We have developed a novel and highly efficient methodology for the synthesis of tetrahydrobenzo[a]-xanthen-11-one and benzo[f]chromen-3-one derivatives from aldehydes, β-naphthol and cyclic 1,3-dicarbonyl compounds or Meldrum's acid. This process is based on the Lewis acid catalytic activity of cerium(III) chloride represents an eco-friendly benign alternative to current chemical processes. We believe that this economically viable procedure could be successfully applied to a variety of cyclic 1,3-dicarbonyl compounds as well as Meldrum's acid to synthesize a variety of one-pot synthesis of xanthene derivatives in excellent yields.
4 Experimental
4.1 General considerations
IR spectra were recorded on Perkin Elmer FTIR-1710 spectrophotometer using KBr pellets. IR frequency, νmax is measured in cm−1. 1H NMR and 13C NMR (400 and 100 MHz, respectively) spectra were recorded on Jeol JNM ECX-400P Spectrometer using TMS as an internal standard. The chemical shift values are recorded on δ scale and the coupling constants (J) are in hertz. Mass spectral data was recorded on Water micromass LCT Mass Spectrometer. The temperature of the reaction mixture was measured through AZ, Mini Gun type, non-contact IR thermometer, model no. 8868. The melting points were determined on Thomas Hoover melting point apparatus. The purity of the compounds was checked on TLC (silica gel coated aluminium sheets, Silica gel 60 F254, E. Merck (Germany).
4.2 General procedure for the synthesis of tetrahydrobenzo[a]-xanthen-11-one, and benzo[f]chromen-3-one derivatives
Aldehydes (1.0 mmol), β-naphthol (1.0 mmol) and 5 mL of methanol were placed in a 50 mL round bottom flask over a magnetic stirrer. Cerium(III) chloride (3 mol%)/(0.03 mmol, 11.12 mg) was added to the mixture and the contents were stirred at 50 °C for an appropriate time. To this stirred mixture, cyclic 1,3-dicarbonyl compounds/Meldrum's acid (1.2 mmol) were added. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was allowed to cool at room temperature and water was added to the reaction mixture and then extracted with ethyl acetate (3 × 10 mL). The organic layer was dried over anhydrous Na2SO4 and evaporated. The crude product was purified by column chromatography (hexane/ethyl acetate, 80:20) to provide the pure products. All the products were synthesized by novel route and elucidated comparing with authentic literature [21d,30,32].
4.3 Spectral data of synthesized compounds (5a–j, 6a–c, 8a–e)
9,9-Dimethyl-12-phenyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5a). White solid; mp 152–153 °C; IR (KBr): 3059, 2960, 2874, 1632, 1379, 1223, 1169, 1075, 812 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.98 (d, J = 8.0 Hz, 1H), 7.70–7.76 (m, 2H), 7.40–7.42 (m, 1H), 7.29–7.38 (m, 4H), 7.14 (t, J = 7.5 Hz, 1H), 7.08 (t, J = 7.4 Hz, 2H), 4.77 (s, 1H), 2.45 (s, 2H), 2.27 (d, J = 17.1 Hz, 1H), 2.21 (d, J = 17.1 Hz, 1H), 1.10 (s, 3H), 0.97 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.1, 165.2, 147.9, 144.8, 130.5, 130.7, 129.1, 128.6, 128.5, 128.3, 127.1, 126.4, 124.7, 123.8, 116.7, 116.1, 113.3, 51.1, 40.6, 34.9, 32.4, 29.5, 27.4; m/z (ESI-MS, HRMS) 355.1653 (M+1, C25H22O2 requires 354.1620).
12-(4-Chlorophenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5b). White solid; mp 183–184 °C; IR (KBr): 3056, 2960, 1602, 1513, 1381, 1217, 1168, 1019, 960, 847, 748 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.97 (d, J = 8.4 Hz, 1H), 7.77 (d, J = 6.1 Hz, 1H), 7.75 (d, J = 8.4 Hz, 1H), 7.39–7.43 (m, 1H), 7.30–7.33 (m, 1H), 7.29 (d, J = 8.7 Hz, 1H) 7.27 (s, 2H), 7.14 (d, J = 8.2 Hz, 2H), 4.73 (s, 1H) 2.55 (s, 2H), 2.56 (s, 2H), 2.45 (d, J = 16.1 Hz, 1H), 2.27 (d, J = 16.1 Hz, 1H), 1.08 (s, 3H), 0.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.4, 164.2, 147.4, 143.5, 131.7, 131.4, 130.1, 129.3, 128.7, 128.8, 127.2, 125.3, 123.7, 117.2, 114.1, 51.6, 41.2, 34.4, 32.5, 29.2, 27.3; m/z (ESI-MS, HRMS) 390.1201 (M+1, C25H21ClO2 requires 388.1230).
12-(4-Bromophenyl)-9,9-dimethyl-8,9,10,12-tetrahydro-benzo[a]-xanthen-11-one (5c). White solid; mp 184–186 °C; IR (KBr): 2959, 2861, 1643, 1591, 1482, 1401, 1373, 1221, 1173, 1144, 1006, 960, 845, 745 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.86 (d, J = 8.3 Hz, 1H), 7.78 (d, J = 6.2 Hz, 1H), 7.74 (d, J = 8.3 Hz, 1H), 7.37–7.42 (m, 1H), 7.29–7.32 (m, 1H), 7.28 (d, J = 8.6 Hz, 1H), 7.26 (s, 2H), 7.13 (d, J = 8.1 Hz, 2H), 4.72 (s, 1H), 2.57 (s, 2H), 2.47 (d, J = 16.3 Hz, 1H), 2.25 (d, J = 16.3 Hz, 1H), 1.05 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.2, 164.3, 147.4, 143.4, 132.1, 131.7, 131.4, 130.1, 129.3, 128.7, 128.6, 127.2, 125.2, 123.6, 117.3, 117.2, 114.1, 51.1, 41.5, 32.4, 29.5, 27.3; m/z (ESI-MS, HRMS) 434.0704 (M+1, C25H21BrO2 requires 432.0725).
9,9-Dimethyl-12-p-tolyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5d). White solid; mp 178–179 °C; IR (KBr): 3044, 2959, 2865, 1610, 1513, 1383, 1219, 1019, 846 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.99 (d, J = 8.4 Hz, 1H), 7.65–7.79 (m, 2H), 7.39–7.41 (m, 1H), 7.30–7.33 (m, 2H), 7.14–7.25 (m, 2H), 7.02 (d, J = 8.0 Hz, 2H), 4.73 (s, 1H), 2.56 (s, 2H), 2.45 (d, J = 16.3 Hz, 1H), 2.22 (d, J = 16.3 Hz, 1H), 2.15 (s, 3H), 1.09 (s, 3H), 0.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.1, 163.2, 153.2, 146.4, 140.6, 134.7, 133.7, 130.4, 128.5, 127.8, 127.1, 126.5, 125.8, 123.9, 122.5, 117.1, 116.5, 115.8, 113.1, 49.6, 40.2, 33.1, 31.1, 28.3, 26.1, 19.4; m/z (ESI-MS, HRMS) 369.1810 (M+1, C26H24O2 requires 368.1776).
12-(4-Methoxyphenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5e). White solid; mp 206–208 °C; IR (KBr): 3018, 2963, 2843, 1677, 12.18, 1161, 1028, 960, 847, 756 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.85 (d, J = 8.4 Hz, 1H), 7.74 (t, J = 9.3 Hz, 2H), 7.41 (t, J = 7.3 Hz, 1H), 7.39 (t, J = 7.2 Hz, 1H), 7.30 (d, J = 8.9 Hz, 1H), 7.25 (d, J = 8.7 Hz, 2H), 6.69 (d, J = 8.6 Hz, 2H), 5.40 (s, 1H), 3.68 (s, 3H), 2.45 (s, 2H), 2.27 (d, J = 16.2 Hz, 1H), 2.21 (d, J = 16.2 Hz, 1H), 1.07 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 197.1, 163.8, 157.9, 147.8, 137.3, 131.6, 129.5, 128.8, 128.5, 127.1, 125.1, 123.9, 118.1, 117.3, 114.7, 113.7, 55.2, 51.3, 41.5, 33.9, 32.4, 29.4, 27.3; m/z (ESI-MS, HRMS) 385.1759 (M+1, C26H24O3 requires 384.1725).
9,9-Dimethyl-12-(4-nitrophenyl)-8,9,10,12-tetrahydrobenzo-[a]-xanthen-11-one (5f). White solid; mp 174–176 °C; IR (KBr): 3075, 2960, 1668, 1652, 1596, 1519, 1347, 1313, 1224, 1165, 960, 832, 756 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.04 (d, J = 8.8 Hz, 2H), 7.79–7.84 (m, 3H), 7.34–7.53 (m, 5H), 4.82 (s, 1H), 2.59 (s, 2H), 2.36 (d, J = 16.5 Hz, 1H), 2.22 (d, J = 16.5 Hz, 1H), 1.11 (s, 3H), 0.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.5, 165.2, 147.6, 143.9, 132.1, 131.8, 131.5, 130.0, 129.3, 128.7, 128.6, 127.3, 125.2, 123.7, 117.13, 117.2, 114.1, 51.2, 41.6, 34.4, 32.5, 29.5, 27.3; m/z (ESI-MS, HRMS) 400.1504 (M+1, C25H21NO4 requires 399.1471).
12-(4-Hydroxyphenyl)-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5 g). White solid; mp 152–153 °C; IR (KBr): 3312, 2956, 1636, 1599, 1381, 1216, 1160, 959, 841, 742 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.77 (d, J = 8.3 Hz, 1H), 7.64–7.66 (m, 2H), 7.42 (t, J = 8.0 Hz, 1H), 7.32 (t, J = 7.4 Hz, 1H), 7.28 (d, J = 8.8 Hz, 1H), 7.14 (d, J = 8.4 Hz, 2H), 6.89 (s, 1H), 6.62 (d, J = 8.4 Hz, 2H), 5.01 (s, 1H), 4.69 (s, 1H), 2.45 (s, 1H), 2.26 (d, J = 16.4 Hz, 1H), 2.22 (d, J = 16.4 Hz, 1H), 1.08 (s, 3H), 0.96 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 198.4, 164.4, 154.2, 147.8, 136.2, 131.6, 131.5, 129.4, 128.9, 128.8, 126.1, 125.2, 123.9, 118.9, 117.2, 115.6, 114.3, 51.2, 41.3, 33.4, 32.6, 29.4, 27.3; m/z (ESI-MS, HRMS) 371.1602 (M+1, C25H22O3 requires 370.1569).
9,9-Dimethyl-12-(naphthalen-2-yl)-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5 h). White solid; mp 234–235 °C; IR (KBr): 3057, 2959, 1693, 1513, 1380, 1218, 1168, 960 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.03 (d, J = 8.3 Hz, 1H), 7.62 (s, 1H), 7.70–7.81 (m, 3H), 7.57 (d, J = 7.7 Hz, 1H), 7.63 (d, J = 8.8 Hz, 1H), 7.42 (d, J = 8.3 Hz, 1H), 7.31–7.40 (m, 5H), 5.78 (s, 1 H), 2.54 (s, 2H), 2.26 (q, J = 16.1 Hz, 2H), 1.12 (s, 3H), 0.94 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 198.2, 163.2, 147.1, 141.3, 133.5, 132.3, 131.7, 131.6, 129.1, 128.6, 128.3, 128.2, 127.6, 127.4, 127.3, 126.9, 125.9, 125.7, 125.1, 123.9, 117.8, 117.3, 114.23, 51.2, 40.6, 34.1, 32.4, 29.5, 27.4; m/z (ESI-MS, HRMS) 405.1810 (M+1, C29H24O2 requires 404.1776).
12-{Benzo[d][1,3]dioxol-5-yl}-9,9-dimethyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5i). White solid; mp 214–215 °C; IR (KBr): 3210, 2960, 1667, 1503, 1361, 1230, 1138, 1039, 960, 847, 748, 621 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.98 (d, J = 8.4 Hz, 1H), 7.79 (d, J = 8.5 Hz, 1H), 7.72 (d, J = 9.0 Hz, 1H), 7.40 (td, J = 7.0, 1.2 Hz, 1H), 7.39 (td, J = 7.9, 0.9 Hz, 1H), 7.34 (d, J = 8.9 Hz, 1H), 6.86 (dd, J = 8.0, 1.7 Hz, 1H), 6.77 (d, J = 1.6 Hz, 1H), 6.62 (d, J = 8.0 Hz, 1H), 5.80 (s, 2H), 4.72 (s, 1H), 2.42 (s, 2H), 2.31 (d, J = 16.4 Hz, 1H), 2.23 (d, J = 16.4 Hz, 1H), 1.06 (s, 3H), 0.98 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 197.5, 164.1, 147.9, 147.7, 146.3, 138.2, 131.7, 131.6, 129.3, 128.6, 127.5, 125.4, 123.9, 121.9, 117.9, 117.3, 114.5, 109.2, 108.3, 101.5, 51.2, 41.6, 34.5, 32.6; m/z (ESI-MS, HRMS) 399.1552 (M+1, C26H22O4 requires 398.1518).
9,9-Dimethyl-12-(thiophen-2-yl)-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (5j). White solid; mp 183–184 °C; IR (KBr): 3124, 2926, 1629, 1513, 1388, 1215, 1171, 1148, 1010, 811, 746 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.78–7.73 (m, 2H), 7.68 (d, J = 8.5 Hz, 1H), 7.29–7.43 (m, 3H), 7.20–7.25 (m, 1H), 7.09–7.16 (m, 2H), 5.57 (s, 1H), 3.68 (s, 2H), 2.45 (s, 2H), 1.25 (s, 3H), 1.07 (s, 3H); 13C NMR (100 MHz, CDCl3): δ = 196.7, 164.5, 148.4, 147.9, 131.5, 129.2, 128.3, 127.1, 126.2, 125.2, 125.1, 124.2, 123.3, 117.3, 117.2, 113.7, 50.8, 41.2, 32.1, 29.3, 29.2, 27.1; m/z (ESI-MS, HRMS) 361.1218 (M+1, C23H20O2S requires 360.1186).
12-Phenyl-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (6a). White solid; mp 191–192 °C; IR (KBr): 3131, 3053, 2956, 1647, 1593, 1453, 1372, 1228, 1190, 998, 956, 817, 759, 702, 532 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.95 (d, J = 8.5 Hz, 1H), 7.76–7.77 (m, 2H), 7.30–7.45 (m, 5H), 7.14–7.17 (m, 2H), 7.03–7.09 (m, 1H), 4.72 (s, 1H), 2.65–2.76 (m, 2H), 2.33–2.48 (m, 2H), 1.95–2.07 (m, 2H); 13C NMR (100 MHz, CDCl3): δ = 197.2, 165.4, 147.9, 145.2, 131.4, 131.5, 128.8, 128.4, 128.5, 128.3, 127.1, 126.2, 124.8, 123.6, 117.8, 117.1, 115.5, 37.2, 34.6, 27.7, 20.2; m/z (ESI-MS, HRMS) 327.1340 (M+1, C23H18O2 requires 326.1307).
12-(4-Chlorophenyl)-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (6b). White solid; mp 208–209 °C; IR (KBr): 3132, 3051, 2960, 1648, 1592, 1487, 1367, 1229, 1190, 1141, 1088, 1008, 955, 817, 754, 531 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.89 (d, J = 8.5 Hz, 1H), 7.77–7.78 (m, 2H), 7.24–7.43 (m, 5H), 7.13–7.15 (m, 2H), 4.71 (s, 1H), 2.62–2.75 (m, 2H), 2.34–2.47 (m, 2H), 1.92–2.07 (m, 2H); 13C NMR (100 MHz, CDCl3): δ = 197.2, 165.7, 147.5, 143.7, 132.1, 131.2, 129.6, 129.3, 128.4, 127.4, 125.6, 123.3, 117.2, 117.1, 115.2, 37.3, 34.3, 27.7, 20.2; m/z (ESI-MS, HRMS) 362.0888 (M+1, C23H17ClO2 requires 360.0917).
12-(4-Nitrophenyl)-8,9,10,12-tetrahydrobenzo[a]-xanthen-11-one (6c). White solid; mp 237–238 °C; IR (KBr): 3076, 2932, 1644, 1596, 1515, 1376, 1342, 1224, 1176, 1025, 961, 847, 752 cm−1; 1H NMR (400 MHz, CDCl3): δ = 8.04 (d, J = 8.4 Hz, 2H), 7.79–7.83 (m, 3H), 7.52 (d, J = 8.9 Hz, 2H), 7.35–7.46 (m, 3H), 4.75 (s, 1H), 2.71–2.80 (m, 2H), 2.37–2.49 (m, 2H), 2.06–2.12 (m, 1H), 1.95–2.01 (m, 1H); 13C NMR (100 MHz, CDCl3): δ = 197.5, 166.3, 152.4, 147.8, 146.3, 131.6, 131.3, 129.9, 129.6, 128.4, 127.6, 125.5, 123.7, 123.3, 117.2, 116.3, 114.4, 37.1, 35.1, 27.9, 20.4; m/z (ESI-MS, HRMS) 372.1191 (M+1, C23H17NO4 requires 371.1158).
1-Phenyl-1H-benzo[f]chromen-3(2H)-one (8a). White solid; mp 117–119 °C; IR (KBr): 3058, 2983, 1735, 1629, 1454, 1212, 1029, 960 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.87 (d, J = 8.5 Hz, 2H), 7.67 (d, J = 8.0 Hz, 1H), 7.39–7.45 (m, 2H), 7.31 (d, J = 6.8 Hz, 1H), 7.24–7.30 (m, 3H), 7.15 (d, J = 7.5 Hz, 2H), 4.92 (dd, J = 7.3, 3.4 Hz, 1H), 3.35–3.40 (m, 2H); 13C NMR (100 MHz, CDCl3): δ = 169.3, 150.1, 140.4, 132.8, 131.2, 130.6, 129.6, 127.9, 127.7, 127.5, 126.9, 124.3, 123.5, 116.7, 116.4, 37.2, 36.8; m/z (ESI-MS, HRMS) 275.1027 (M+1, C19H14O2 requires 274.0994).
1-(4-Chlorophenyl)-1H-benzo[f]chromen-3(2H)-one (8b). Colourless oil; IR (Film): 3058, 2955, 1738, 1630, 1436, 1213, 1173, 1092, 960, 847, 749 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.70–7.98 (m, 2H), 7.67 (d, J = 8.2 Hz, 1H), 7.39–7.43 (m, 2H), 7.32 (d, J = 8.6 Hz, 1H), 7.22 (d, J = 8.3 Hz, 2H), 7.10 (d, J = 7.8 Hz, 2H), 4.91 (d, J = 5.8 Hz, 1H), 3.76 (dd, J = 10.8, 2.4 Hz, 1H), 3.53 (dd, J = 12.5, 3.0 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ = 167.2, 150.9, 140.2, 134.7, 132.2, 130.2, 129.7, 128.9, 128.5, 127.3, 126.1, 124.2, 117.8, 116.9, 37.5, 36.8; m/z (ESI-MS, HRMS) 309.1865 (M+1, C19H13ClO2 requires 308.0604).
1-(4-Fluorophenyl)-1H-benzo[f]chromen-3(2H)-one (8c). White solid; mp 136–138 °C; IR (KBr): 3061, 2955, 1733, 1654, 1516, 1347, 1270, 1173, 1072, 960, 851, 748 cm−1; 1H NMR (400 MHz, CDCl3) δ = 8.30 (d, 8.5 Hz, 1H), 7.61 (d, J = 8.0 Hz, 1H), 7.51 (d, J = 8.0 Hz, 1H), 7.34–7.37 (m, 2H), 7.31 (d, 8.0 Hz, 1H), 7.24–7.27 (m, 2H), 7.05–7.10 (m, 2H), 4.80 (d, J = 5.9 Hz, 1H), 3.77 (dd, J = 14.6, 3.4 Hz, 1H), 3.16 (dd, J = 14.2, 2.5 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ = 169.2, 161.0, 148.7, 137.3, 136.9, 132.4, 130.2, 129.3, 128.6, 127.4, 127.0, 125.8, 122.1, 118.6, 117.5, 116.3, 116.2, 37.5, 36.7; m/z (ESI-MS, HRMS) 293.6101 (M+1, C19H13FO2 requires 292.0900).
1-(4-Methoxyphenyl)-1H-benzo[f]chromen-3(2H)-one (8d). Yellowish solid; mp 135–136 °C; IR (KBr): 3060, 2920, 1727, 1635, 1438, 1213, 1138, 1014, 958, 843, 742 cm−1; 1H NMR (400 MHz, CDCl3): δ = 7.79 (d, J = 8.2 Hz, 2H), 7.77 (d, J = 8.2 Hz, 1H), 7.64–7.71 (m, 2H), 7.24 (d, J = 8.6 Hz, 1H), 7.09 (d, J = 7.8 Hz, 2H), 6.94 (d, J = 8.7 Hz, 2H), 4.78 (dd, J = 6.6, 3.2 Hz, 1H), 3.70 (s, 3H), 3.23 (dd, J = 12.6, 2.8 Hz, 1H), 3.08 (dd, J = 12.7, 3.4 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ = 168.3, 157.0, 148.2, 134.8, 132.6, 131.3, 129.2, 128.4, 127.8, 127.6, 126.8, 125.4, 123.5, 1119.0 117.30, 115.2, 114.1, 56.3, 37.9, 35.8; m/z (ESI-MS, HRMS) 305.1133 (M+1, C20H16O3 requires 304.1099).
1-(Thiophen-2-yl)-1H-benzo[f]chromen-3(2H)-one (8e). Sticky; IR (Film): 2954, 1716, 1602, 1436, 1208, 1120, 1070, 960, 845, 745 cm−1; 1H NMR (400 MHz CDCl3): δ = 7.74 (d, J = 7.8 Hz, 1H), 7.64–7.71 (m, 2H), 7.38–7.52 (m, 3H), 7.20–7.30 (m, 1H), 7.08–7.14 (m, 2H), 4.01 (s, 1H), 3.92 (dd, J = 12.6, 2.2 Hz, 1H), 3.79 (dd, J = 12.6, 2.5 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ = 169.1 147.3, 144.2, 134.6, 128.6, 127.8, 127.0, 126.7, 126.3, 125.5 125.3, 125.1, 123.3, 122.4, 37.6, 35.7; m/z (ESI-MS, HRMS) 281.0592 (M+1, C17H12O2S requires 280.0558).
Acknowledgements
A. Jahan thanks to University Grants Commission (UGC) for the grant of Junior Research Fellowship and N. K. Mishra is grateful to CSIR, New Delhi, India for the award of Research Associateship. The authors also would like to acknowledge the University Science Instrumentation Centre (USIC), University of Delhi for providing the instrumental facilities to characterize the synthesize compounds.


