1 Introduction
Benzo[b]pyrans and their derivatives constitute an important class of organic compounds due to their attractive pharmacological and biological properties [1,2]. They are widely used as anticoagulant, anticancer, diuretic, spasmolitic and antianaphylactin agents [3,4] and can be used as cognitive enhancers for the treatment of neurodegenerative disease, including Alzheimer's disease, Huntington's disease, Parkinson's disease and Down's syndrome as well as for the treatment of schizophrenia and myoclonus [5,6]. 4H-pyrans occur in a series of natural products [7,8] and some of 2-amino-4H-pyrans have photochemical activities [9]. Consequently, many methods for the synthesis of these compounds have been reported, including the use of microwave [10], ultrasonic irradiations [11] and a variety of reagents like sodium bromide [10], hexadecyldimethyl benzyl ammonium bromide [12], tetramethyl ammonium hydroxide [13], diammonium hydrogen phosphate [14], fluoride ion [15], magnesium oxide [16], sodium selenate [17], iodine [18], H6P2W12O62.H2O [19], tetrabutylammonium bromide [20], cerium(III) chloride [21], lithium bromide [22], Amberlite IRA-40 (OH−) [23], acidic ion liquids [24], l-proline [25], ZnO-beta Zeolite [26], trisodium citrate [27], and basic ionic liquids [28].
Arylboronic acids have been used as catalysts in several reactions such as formation of ethers [29], 1,3-transposition of allylic alcohols [30], Diels Alder cycloadditions [31], [3+2] dipolar cycloaddition [32] and amidation of carboxylic acids [33–35].
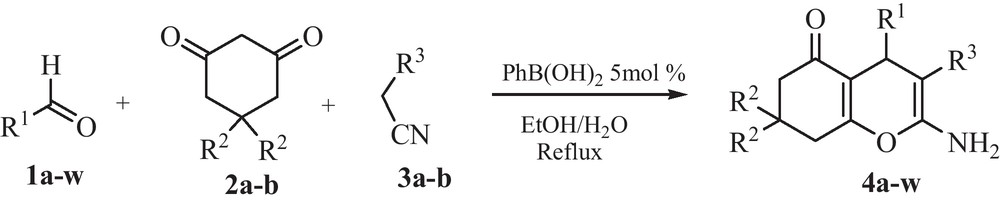
Phenylboronic acid, a commercially available material, has been exploited previously by us in Biginelli and Hantzsch three-component reactions [36,37] as a nontoxic, inexpensive, easy handling and mild catalyst. Now we wish to report here the catalytic activity of PhB(OH)2 in the one-pot synthesis of tetrahydrobenzo[b]pyrans 4 between aromatic aldehydes 1, dimedone or 1,3-cyclohexanedione 2 and malonitrile (or ethyl cyanoacetate) 3 in refluxing H2O/EtOH (Scheme 1).
2 Results and discussion
In order to optimize the conditions, we studied the reaction of benzaldehyde 1a with dimedone 2a, malonitrile 3, and 20 mol % of phenylboronic acid as a simple model substrate in various conditions. First, we tested the effect of various solvents at different temperatures. When using aprotic polar solvent such as CH3CN, the reaction afforded the corresponding 4H-benzopyran 4a with a modest yield of after 6 h at reflux (Table 1, entry 1). However, the reactions in refluxing protic solvents such as H2O or EtOH gave better yields (entries 2 and 3); nevertheless, it was not completed even after 3 days at ambient temperature (entry 4). Similarly, the reaction without any solvent at 80 °C was not very successful (entry 5). The 50% aqueous ethanol is proven to be the most suitable solvent for this condensation in terms of yield and reaction time (entry 6).
Optimization of the reaction using different conditions.a
| Entry | Solvent | Catalyst (mol %) | Time (h) | Temperature (°C) | Yieldb (%) |
| 1 | CH3CN | PhB(OH)2 20 | 6 | Reflux | 38 |
| 2 | H2O | PhB(OH)2 20 | 1 | Reflux | 75 |
| 3 | EtOH | PhB(OH)2 20 | 3 | Reflux | 61 |
| 4 | EtOH | PhB(OH)2 20 | 72 | Ambient | 10 |
| 5 | None | PhB(OH)2 20 | 6 | 80 | 54 |
| 6 | EtOH/H2O | PhB(OH)2 20 | 0.5 | Reflux | 85 |
| 7 | EtOH/H2O | PhB(OH)2 10 | 0.5 | Reflux | 88 |
| 8 | EtOH/H2O | PhB(OH)2 5 | 0.5 | Reflux | 88 |
| 9 | EtOH/H2O | PhB(OH)2 50 | 0.5 | Reflux | 83 |
| 10 | EtOH/H2O | PhB(OH)2 100 | 0.5 | Reflux | 83 |
| 11 | EtOH/H2O | Ph-CH=CH-B(OH)2 5 | 0.5 | Reflux | 85 |
| 12 | EtOH/H2O | CeCl3.7H2O 5 | 0.5 | Reflux | 57 |
a The reactions were conducted by condensation of benzaldehyde 1a (1 equiv.), dimedone 2a (1 equiv.), and malonitrile 3 (1 equiv.).
b Isolated yields.
We also evaluated the amount of phenylboronic acid required for the reaction. It was found that when decreasing the amount of the catalyst from 20 to 10 mol %, the yield increased from 85 to 88% (entry 7). The use of 5 mol% of PhB(OH)2 maintaining the yield at 88%, so this amount is sufficient to promote the reaction. In the presence of more than this amount of the catalyst, neither the yield nor the reaction time were improved (entries 9 and 10). Thus, the best result was obtained with 5 mol % of catalyst in 50% aqueous ethanol at reflux (entry 8).
In comparison with PhB(OH)2, the use of Ph-CH=CH-B(OH)2 as catalyst in the model reaction under refluxing condition showed good catalytic effects and afforded comparable yield of the desired product (entry 11).
In contrast, other Lewis acid catalysts such as CeCl3.7H2O gave lower yield (entry 12).
All reactions delivered good to excellent products yields and accommodated a wide range of aromatic aldehydes containing electron-donating and electron-withdrawing groups (entries 1–12) without any significant substituent effect. This three-component condensation reaction also proceeded with heteroaromatic aldehyde, such as 2-furaldehyde and give the corresponding product in high yield (entry 13). The scope of this one-pot reaction was further extended by replacing dimedone 2a with cyclohexane-1,3-dione 2b and various highly functionalized 4H-benzo[b]pyrans were produced in good yields (entries 14–16). However, aliphatic aldehydes such as acetaldehyde, propionaldehyde and isobutyraldehyde needed longer reaction times to provide moderate yields of the corresponding products (entries 17–19).
To assess the generality and versatility of the catalyst, the same reaction conditions as described above were applied for the synthesis of ethyl 2-amino-4H-benzo[b]pyrans-3-carboxylate by replacing malonitrile 3a with ethyl cyanoacetate 3b. The expected compounds were then obtained in good yields (entries 20–21).
In all cases, the final products were isolated by simple filtration, washed with cold water and purified by recrystallization from ethanol1.
We propose the following mechanism to account for the reaction. We checked that, at reflux for 30 min in EtOH/H2O (1/1) without phenylboronic acid, malonitrile reacts quite quantitatively with aromatic aldehyde 1 in a Knoevenagel transformation, while only small amounts of 4 was produced in such conditions. 1,3-Dicarbonyl compound would be therefore first activated by phenylboronic acid to give a boron enolate 2’. Addition to arylidenemalonitrile 5 would lead to the formation of 6. After a spontaneous deborylation that usually occurs when a boronated group is in an α-position to a cyano or keto group, tautomerism and intramolecular cyclization afforded the final product 4 (Scheme 2).
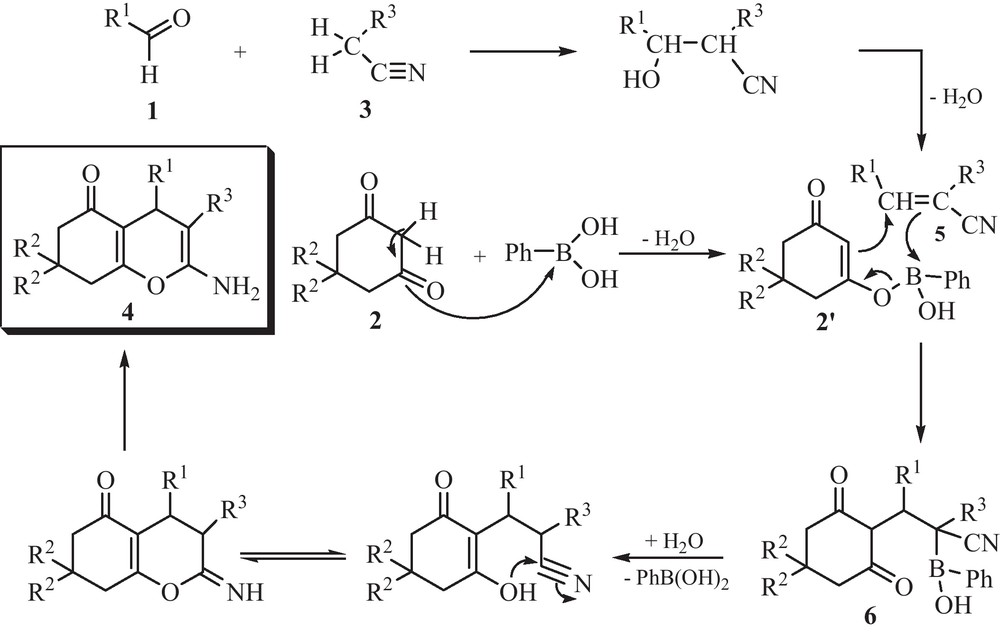
With the above results, we can realize that the phenylboronic acid catalyses only the second step of the reaction (Table 2).
Synthesis of tetrahydrobenzo[b]pyrans 4a–w by condensation of aldehydes, malonitrile or ethyl cyanoacetate and dimedone or 1,3-cyclohexanedione using PhB(OH)2 as catalyst in refluxing H2O:EtOH (1:1).
| Entry | R1 | R2 | R3 | Time (h) | Product | Yield (%)a | Mp (°C) Measured | Mp (°C) Reported |
| 1 | C6H5 | Me | CN | 0.5 | 4a | 88 | 236–238 | 234–235 [15] |
| 2 | 4-Me-C6H4 | Me | CN | 0.5 | 4b | 65 | 215–218 | 210–212 [13] |
| 3 | 4-MeO-C6H4 | Me | CN | 0.5 | 4c | 87 | 204–205 | 200–201 [26] |
| 4 | 2-MeO-C6H4 | Me | CN | 0.5 | 4d | 81 | 194–196 | 195–197 [38] |
| 5 | 4-(NO2)-C6H4 | Me | CN | 0.5 | 4e | 88 | 180–182 | 177–178 [26] |
| 6 | 3-(NO2)-C6H4 | Me | CN | 0.5 | 4f | 85 | 210–212 | 213–215 [16] |
| 7 | 4-(OH)-C6H4 | Me | CN | 0.5 | 4g | 84 | 216–218 | 213–214 [26] |
| 8 | 4-(Br)-C6H4 | Me | CN | 0.5 | 4h | 86 | 199–200 | 207–208 [26] |
| 9 | 4-(Cl)-C6H4 | Me | CN | 0.5 | 4i | 84 | 207–209 | 209–210 [26] |
| 10 | 3-(Cl)-C6H4 | Me | CN | 0.5 | 4j | 70 | 228–229 | 230–232 [13] |
| 11 | 4-Me2N-C6H4 | Me | CN | 0.5 | 4k | 86 | 218–220 | 217–218 [15] |
| 12 | C6H5-CH=CH- | Me | CN | 0.5 | 4l | 74 | 208–210 | 205–207 [39] |
| 13 | 2-Furyl | Me | CN | 0.5 | 4m | 85 | 222–224 | 226–228 [15] |
| 14 | 4-MeO-C6H4 | H | CN | 0.5 | 4n | 72 | 198–200 | 190–192 [16] |
| 15 | 4-(NO2)-C6H4 | H | CN | 0.5 | 4o | 76 | 240–241 | 235–237 [16] |
| 16 | 4-(Cl)-C6H4 | H | CN | 0.5 | 4p | 61 | 229–230 | 225–227 [16] |
| 17 | CH3 | Me | CN | 2 | 4q | 43 | 177–179 | 171–173 [40] |
| 18 | CH3CH2 | Me | CN | 2 | 4r | 41 | 193–194 | 190–194 [17] |
| 19 | (CH3)2CH | Me | CN | 2.5 | 4s | 38 | 154–156 | 156–157 [40] |
| 20 | 3-(NO2)-C6H4 | Me | CO2Et | 1 | 4t | 80 | 170–172 | 179–181 [13] |
| 21 | 4-(Cl)-C6H4 | Me | CO2Et | 1 | 4u | 65 | 155–157 | 157–159 [13] |
a The reaction was conducted in a non-toxic conditions.
3 Conclusion
In conclusion, we have reported an easy, convenient, inexpensive and friendly environmental synthetic approach for the preparation of tetrahydrobenzo[b]pyrans catalyzed by PhB(OH)2 using a three-component condensation in aqueous ethanol. This procedure offers advantages like high yields, operational simplicity, non-toxic catalyst and solvents, short reaction time and minimum pollution of the environment, which makes it a useful and attractive process for the preparation of these compounds.
1 2-Amino-7,7-dimethyl-4-ethyl-5-oxo-5,6,7,8-tetrahydro-4H-benzopyran-3-carbonitrile (4s). IR (KBr, cm−1) ν: 3417, 2191, 1654, 1380; 1H NMR (250 MHz, DMSO-d6) δ: 6.16 (s, 2H), 3.20 (t, J = 4.4 Hz, 1H), 2.31 (s, 2H), 2.17 (s, 2H), 1.53–1.38 (m, 2H), 1.03 (s, 3H), 1.01 (s, 3H), 0.69 (t, J = 7.2 Hz, 3H); 13C NMR (62.9 MHz, DMSO-d6) δ: 201.30, 168.06, 164.90, 125.05, 117.63, 61.31, 55.50, 36.70, 35.08, 33.87, 32.71, 32.09, 31.77.


