1 Introduction
Diamond presents a number of outstanding properties, attractive for many applications as electronic devices, field emitters, high temperature sensors, biomedical applications, and for preparing very stable electrodes for electrochemistry (see, for example, the patent from [1]) [2]. The surface termination is one of the most important parameters affecting the properties of diamond. It is well reported that hydrogen-terminated surfaces are hydrophobic and have a high conductivity, whereas oxygen-terminated ones are hydrophilic and have a low conductivity [3–5].
Even if as-deposited hydrogen terminations are highly stable on diamond surfaces, they can be converted to oxygen terminations, either by dry (UV, O2 plasma…) [3–13] or wet techniques (anodic treatments…) [14–18]. Despite many works being devoted to the oxidation of diamond surfaces, it is still a challenge to successfully produce well-defined “C-O” functions, particularly for functionalization purposes. Oxygen terminations such as COH, C=O or COOH exhibit a higher potential for functionalization, compared to ether bridges (COC). Indeed, well-known chemical routes can be used to link functional groups to diamond surfaces with hydroxyl, carbonyl or carboxylic species. These highly reactive species are then the most desired ones when oxidation treatments are performed on diamond.
“Electroless” oxidation has been widely described on III-V and II-VI semiconductor (SC) interfaces. These works showed that using oxidizing agents with standard potentials close to the valence band (VB) position of the material, the reduction of the oxidizing agent by holes injection and the oxidation of the SC take place simultaneously on the same surface [19–23]. In a previous work, the “electroless” oxidation of as-deposited polycrystalline boron-doped diamond (BDD) films, using four different oxidizing solutions at room temperature, Ce4+, MnO4− and S2O82− in H2SO4 and H2O2 30% was investigated [24]. The resulting surfaces were characterized using contact angle measurements and X-ray photoelectron spectroscopy, evidencing an efficient oxidation method of the diamond surface. In the present work, the reduction mechanisms of the oxidizing agents at the diamond surface were studied. Current-voltage measurements were performed using a rotating disk electrode (RDE) of BDD immersed in a solution containing one of the species. Different rotation speeds were applied and two different kinds of mechanisms were evidenced.
2 Experimental
2.1 Materials
Cerium sulfate (Ce(SO4)2), potassium permanganate (KMnO4), hydrogen peroxide (H2O2) and ammonium persulfate ((NH4)2S2O8) were purchased from Aldrich and used without further purification.
Sulfuric acid (H2SO4) was provided by Prolabo (Normapur quality).
The polycristalline BDD films, deposited on silicon substrates in a hot filament-assisted chemical vapor deposition process, were provided by Adamant (Neuchatel, Switzerland). The concentration of boron atoms in the diamond film was determined to be 1 × 1020 B.cm−3 by SIMS measurements. Because of the deposition process, the surface is mainly hydrogenated and samples will be termed H-BDD.
2.2 Current-voltage measurements using a rotating disk electrode
Current-voltage measurements were performed in either Ce4+, MnO4−, S2O82− or H2O2 mM solutions in H2SO4 0.5 M using an EG&G 273 potentiostat and a Tacussel electronic CTV101 T RDE. Electrochemical experiments were performed using a classical three-electrode device with a mercury sulfate electrode (MSE) as reference, H-BDD as working and platinum (for the experiments in Ce4+, MnO4− and S2O82−) or gold (for the experiments in H2O2, because of the catalysis of H2O2 dismutation by platinum) wire as counter electrode.
3 Results and discussion
In our previous work [24], H-BDD samples were treated in solutions containing one of the four oxidizing agents Ce4+, MnO4−, S2O82− or H2O2. The concentrations of oxidizers were chosen close to their limit solubility. Based on concepts developed for classical III-V electrochemistry, oxidants were chosen because of the good energetic overlap between their standard redox potentials and the VB edge of diamond (Fig. 1), allowing a good hole injection in the eventuality of an electrochemical oxidation process. As evidenced on Fig. 1, the VB edge position of H-BDD samples determined by Mott-Schottky measurement [27] is set at 1.0 V/MSE. The surface wettability was followed by contact angle measurements (Table 1). On as-grown diamond, the contact angle with water is about 100°, due to hydrophobic “C-H” terminations [25–27]. After the oxidation process with Ce4+, MnO4−, S2O82− or H2O2 solutions, the contact angle with water decreased, due to the formation of hydrophilic “C-O” groups, which was confirmed by XPS analyses, as described in [24] and summerized in Table 1.
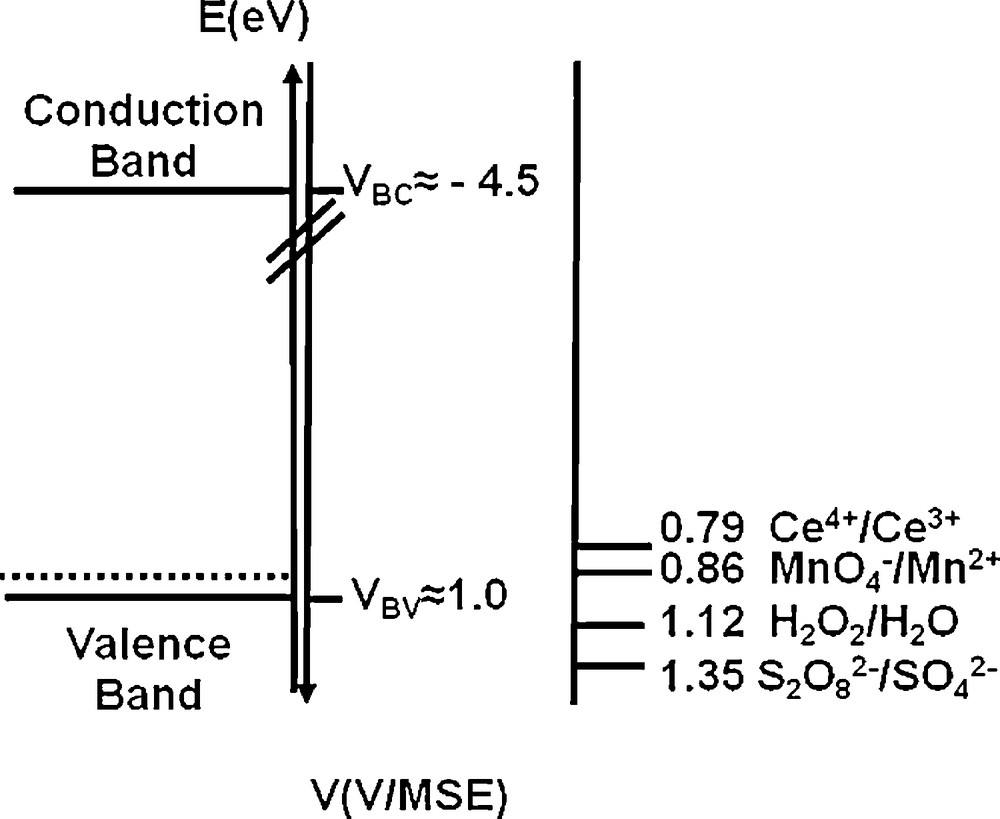
Energetic diagram of H-BDD in H2SO4 0.5 M and position of the standard potentials of the redox couples: Ce4+/Ce3+, MnO4−/Mn2+, H2O2/H2O and S2O82−/SO42−.
Contact angles with water, oxygen amounts and proportions of the different oxygen-containing groups before and after chemical oxidations in Ce4+, MnO4−, S2O82− and H2O2.
| Contact angle with water (°) | [O]/[C] (%) | C-O (%) (C-OH and C-O-C) | C=O (%) | COOH (%) | |
| H-BDD | 100 | 5 | 4.5 | 0 | 0.5 |
| O-BDD (1 week Ce4+) | 40 | 20 | 16.5 | 2.5 | 1 |
| O-BDD (1 week MnO4−) | 60 | 18 | 14.5 | 2.5 | 1 |
| O-BDD (1 week H2O2) | 60 | 9 | 8.5 | 0 | 0.5 |
| O-BDD (1 week S2O82−) | 60 | 9 | 8.5 | 0 | 0.5 |
Whatever the specie used in the open current potential (OCP) process, the oxidation of diamond surfaces takes place, but the efficiency of the process seems dependent on the oxidizing agent. Ce4+ and MnO4− appear to be particularly efficient, the oxygen amount going from 5% on an as-grown sample to about 20% after a 48-hour treatment, while the maximum quantity of oxygen terminations only raises up to 9% with OCP treatments in presence of persulfate or hydrogen peroxide even for durations of more than 1 week [24].
As different oxidation efficiencies are observed with the four oxidants, different mechanisms have been suggested. Hence, to investigate the oxidation mechanisms of diamond surfaces through these OCP processes, the study of the reduction of the different oxidizing agents at the diamond surface was undertaken. With this aim, current-voltage measurements were performed using a H-BDD RDE immersed in a solution containing one of the four species either Ce4+, MnO4−, S2O82− or H2O2 mM in H2SO4 0.5 M. Different rotation speeds were studied (Figs. 2 and 6).
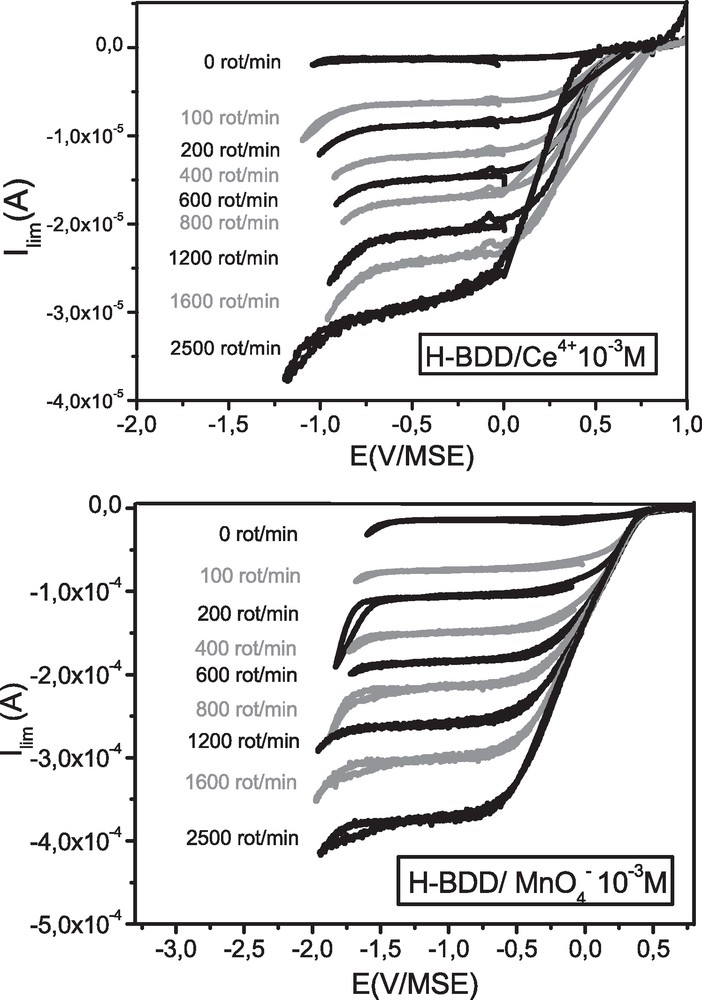
Current-voltage curves for H-BDD in Ce4+ and MnO4− mM/H2SO4 0.5 M for different rotation (rot) speeds of the rotating disk electrode.
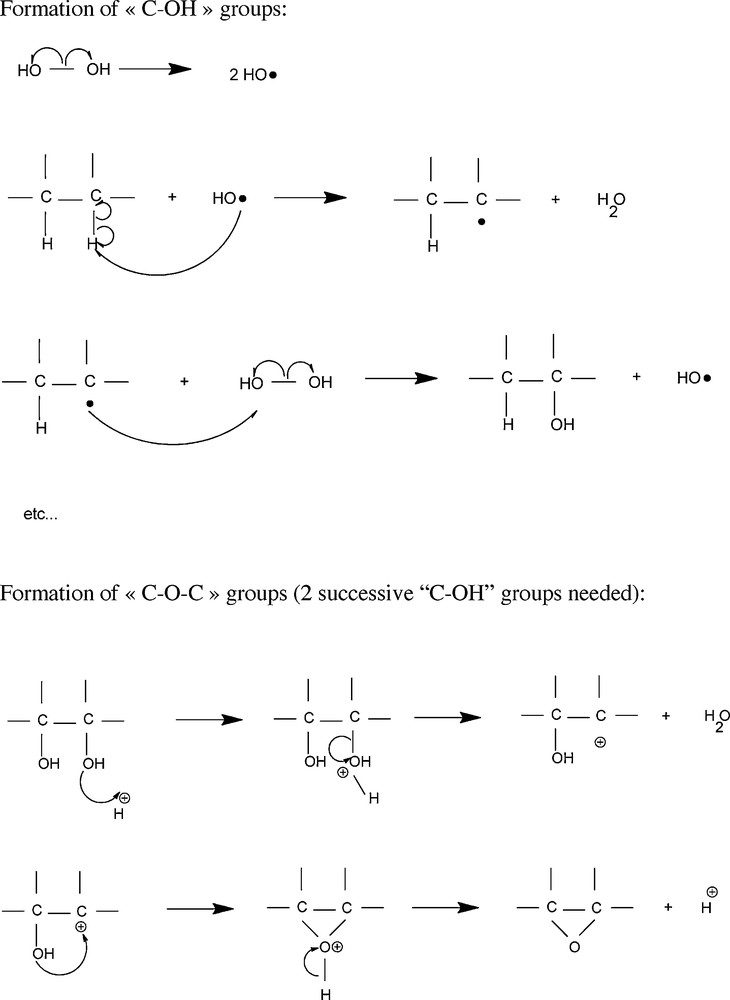
Model for the chemical oxidation of BDD with H2O2.
3.1 Rotating disk electrode (RDE) experiments with ceric and permanganate
With Ce4+ and MnO4−, reduction plateaus depending on the rotation speed of the diamond electrode are observed (Fig. 2). Hence, the reduction processes are dependent on the diffusion of the oxidizing species as already reported for ceric ion by Kelly et al. [28].
The limit diffusion currents (Ilim) depending on the rotation speed of the electrode were determined from the reduction plateaus. Levich plots (|Ilim| = f(v1/2), v being the rotation speed of the electrode) were drawn and reported (Fig. 3). With both Ce4+ and MnO4−, perfectly straight lines passing through the origin are obtained. In these conditions, a purely charge transport limited current is highlighted.

Levich plots for H-BDD rotating electrodes in Ce4+ and MnO4− mM/H2SO4 0.5 M.
So, RDE results evidence that the reduction of Ce4+ and MnO4− at BDD surfaces takes place through an electrochemical process. As shown by the Levich plots, the process is a purely diffusion controlled one (on the plateaus), which strongly suggests that the charge transfer between the diamond surface and the oxidizing agent is very efficient, even if the energetic overlaps between the SC VB and the standard potentials of the two redox couples (Ce4+/Ce3+, MnO4−/Mn2+) are good but not optimal. As suggested by Kelly et al. in the case of Ce4+/Ce3+ couple [28], surface states located in the gap of diamond could mediate charge transfer between the VB and the redox system, which could also explain our results.
As singled C-O bonds are mainly grown on H-BDD surfaces in contact with Ce4+ or MnO4− (Table 1), it has been suggested [24,27] that the first step in the oxidation of BDD surfaces with electroless treatment is the hole injection in the VB from the reduction of the oxidizing agent (Eq. (1a)), leading to the break of C-H bonds and forming a radical site on the diamond surface (Eq. (1b)):
| (1a) |
| (1b) |
As already described [23,26] two reactions are possible after this first step including a two- and/or a four-injection holes mechanism (Fig. 4), leading to the formation of either hydroxyl and/or ether groups on BDD surface. The four hole process leads to the creation of C-O-C terminations (5-a): two first holes are used to produce two successive C radicals at the diamond surface, then in a second step, two H2O molecules and two other holes are required to create a C-O-C group. Differently, the two holes process (5-b) leads to the creation of C-OH terminations: a first hole is used to produce a C radical at the diamond surface then, a second hole and a water molecule are required to form a C-OH group.

Model for the electroless oxidation of BDD by hole injection in the valence band of the semiconductor (2- and 4-holes models).
Similar oxygen amounts are measured after OCP treatments with MnO4− or Ce4+ (Table 1), showing that oxidation efficiency is very similar with both oxidizers. As observed on Fig. 2, the diffusion current corresponding to the reduction of the permanganate ion is much higher compared to that of the ceric specie, in agreement with a reduction mechanism involving five times more charges in the case of MnO4− compared to Ce4+. Our results suggest that even if a more important hole injection is involved in the case of permanganate, the oxidation efficiency is similar with both oxidizers, suggesting a predominance of the four-hole mechanism (5-a) for treatment with MnO4− and a two-hole (5-b) for treatment with ceric ion.
This hypothesis is supported by the values of contact angles with water measured after the two treatments: 40° for Ce4+ and 60° for MnO4−, with same amounts of oxygen at the BDD surface [24]. Indeed, wettability measurements suggest that more polar terminations are created in the case of Ce4+, which is in agreement with the two-injection holes mechanism which gives a majority of “C-OH” groups. Besides, a more important contact angle with water suggests the presence of less polar terminations in the case of MnO4− which is in agreement with the formation of a greater part of “C-O-C” obtained by the four-injection holes mechanism.
These two models could explain the differences observed after “electroless” processes with ceric or permanganate oxidizers, but further investigations are needed to check these hypotheses.
3.2 Rotating disk electrode (RDE) experiments with hydrogen peroxide and persulfate
To investigate the oxidation processes involved in the cases of H2O2 and S2O82−, similar RDE experiments have been made to study the reduction of these two oxidants at diamond surface.
In both cases, the reduction current is independent of the rotation speed of the electrode and close to zero (Fig. 5), suggesting that charge transfer does not occur between diamond surface and H2O2 or S2O82− species, despite a good energetic overlap (Fig. 1). As the growth of C-O terminations is evidenced after OCP treatments with these oxidants, a chemical oxidation should take place at the diamond surface. With hydrogen peroxide, despite a very good overlap with the SC, Kelly et al. [19] have also reported such chemical process for the OCP oxidation of GaAs or InP.
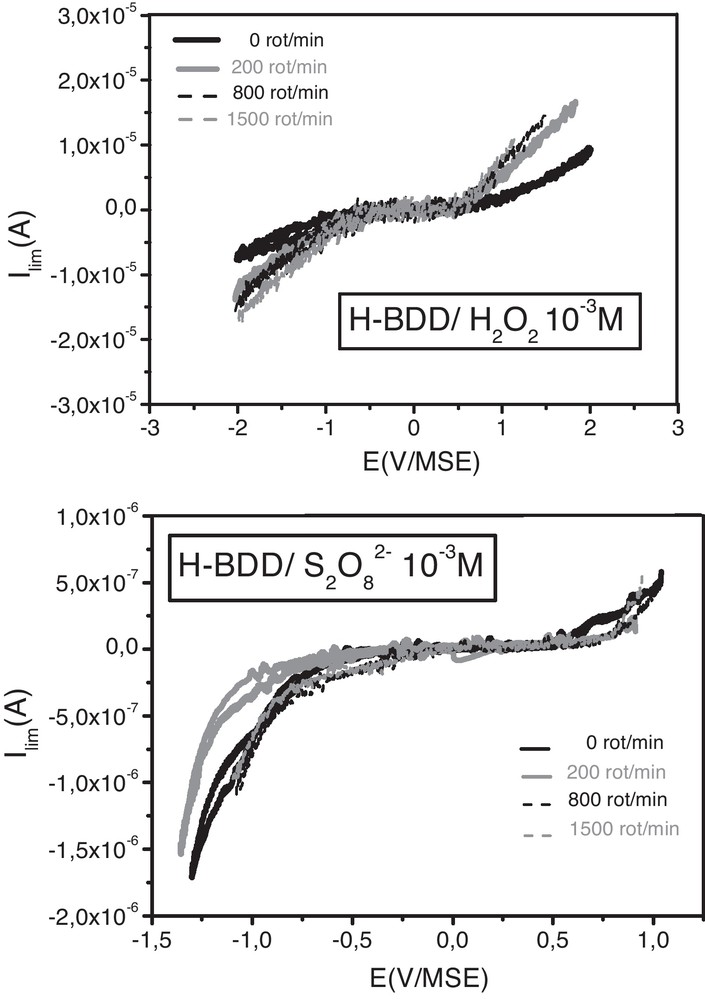
Current-voltage curves for H-BDD in H2O2 and S2O82− mM/H2SO4 0.5 M for different rotation (rot) speeds of the rotating disk electrode.
A model is proposed on Fig. 6 for the chemical oxidation of diamond by H2O2. Due to light exposure, the first step may be the homolytical cleavage of the weak oxygen-oxygen bond of the hydrogen peroxide molecule, producing very reactive hydroxyl radicals [29–31]. Then the second step could be the homolytical cleavage of the C-H bond at the diamond surface accompanied by the formation of a stable H2O molecule and a C radical. Finally, the C radical could react with either a hydroxyl radical or another hydrogen peroxide molecule to form a C-OH bond. When two successive C-OH groups are observed, a first hydroxide may catch a proton in the medium and lead to the creation of a H2O molecule and a carbocation at the diamond surface. The second hydroxide group could attack the carbocation to form a C-O-C termination accompanied by the release of a proton.
In the present study, H-BDD samples were immerged in peroxide solution simply kept under natural light. To check the model, based on the production of hydroxyl radicals under light, new samples were exposed to the same hydrogen peroxide solution 30% in the dark. In this case, no increase of the oxygen amount was observed by XPS measurements, meaning that the oxidation process is inefficient under dark conditions. This result confirms the mechanism proposed to explain the chemical oxidation with H2O2. Additionally, authors [32] showed that, in the dark, hydroxyl radicals were formed at pH 6.5 and above but were not produced at pH 5 and below which corresponds to our oxidizing solution (30% H2O2). To conclude, light is requested for the first step to occur in the chemical oxidation of BDD surfaces with hydrogen peroxide, supporting our mechanism.
A second mechanism is proposed on Fig. 7 to explain the chemical oxidation of diamond by S2O82−. Here as well, due to light exposure, the first step may be the homolytical cleavage of the oxygen-oxygen bond of the persulfate molecule, producing highly reactive sulfate radicals [33]. The second step could be the homolytical cleavage of the C-H bond at the diamond surface accompanied by the formation of a C radical. At the same time, hydroxyl radicals could be produced by the reaction between a sulfite radical and a water molecule. Finally, the C radical could react with a hydroxyl radical to form a C-OH bond. The formation of “C-O-C” groups would be possible when two successive “C-OH” are produced, exactly as previously explained for H2O2.
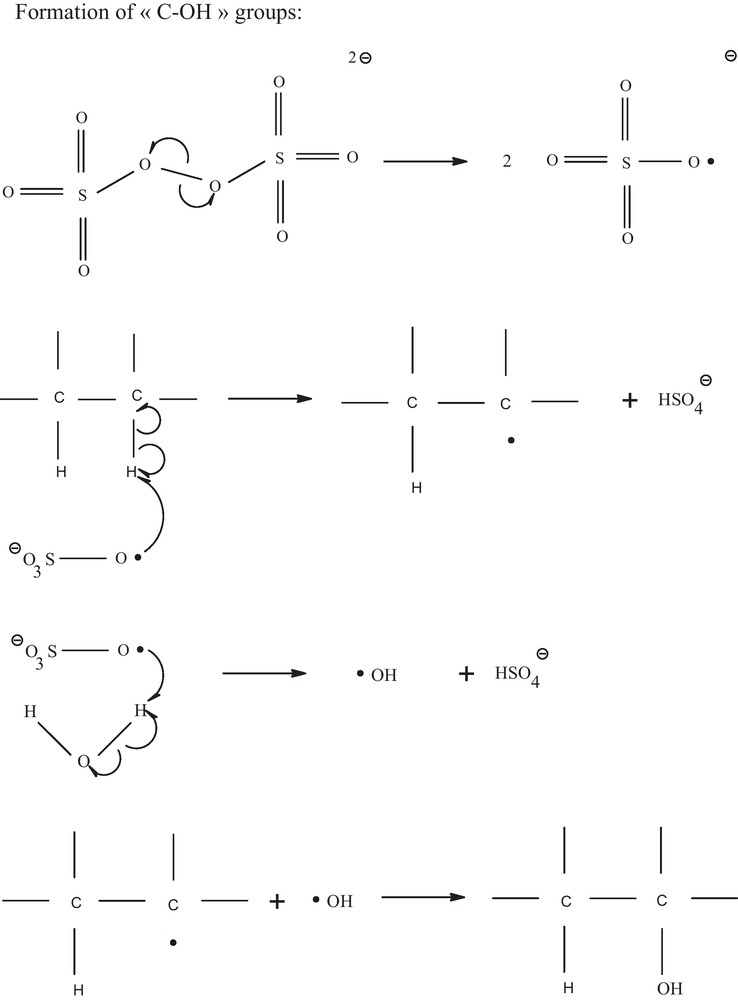
Model for the chemical oxidation of BDD with S2O82−.
Here as well, to check the model based on the production of radicals under light exposure, similar experiments were performed in the dark. No increase of the oxygen amount was observed by XPS measurements, showing that the oxidation process is inefficient in the dark and confirming our model.
Finally, two different mechanisms were evidenced for the OCP oxidation of diamond surfaces: an electrochemical one with Ce4+ and MnO4− leading to approximately 20% of oxygen-terminated functions and a chemical one with H2O2 and S2O82−, leading to about 9% of “C-O”. The two different mechanisms are thus related to different oxidation efficiencies. A previous work [34] has already underlined the benefit of electrochemical anodic treatments compared to plasma ones for the growth of oxygen groups on BDD. Here again, as anodic processes, the electrochemical oxidation shows a superiority in terms of efficiency with additional advantages as the ease to perform and the adaptability to non-conductive diamond surfaces.
4 Conclusion
In this work, the oxidation mechanism of diamond surfaces with four oxidizing agents at OCP conditions was investigated.
RDE experiments show that the reduction of oxidizers takes place at BDD surfaces either via a chemical process for H2O2 and S2O82− or via an electrochemical mechanism for Ce4+ and MnO4−.
Mechanisms have been proposed to explain the formation of C-O functions, either ether or alcohol, at BDD surface with the different oxidizers. The first step in the oxidation mechanism with Ce4+ and MnO4− ions should be the break of C-H bonds due to hole injection in the diamond VB, leading to the formation of radical sites C· at BDD surface. Differently, chemical oxidation with hydrogen peroxide or peroxodisulfate seems to be based on the production due to light exposure of radicals who react with the BDD surface.
Finally, XPS analysis shows that the two different mechanisms are related to different oxidation efficiencies, the electroless oxidation leading to a larger amount of oxygen groups at diamond surface than the chemical one.


