1 Introduction
Multicomponent reactions (MCRs) are one of the most important concepts in modern synthetic organic chemistry. MCRs have received significant demand as a direct route for the synthesis of diverse compounds and compound libraries [1–5]. For example, multicomponent Cu(I)-catalyzed 1,3-dipolar azide–alkyne cycloaddition (CuAAC) leads to a diversity of 1,4-disubstituted 1,2,3-triazole compounds; this is best known as the click reaction [6]. Many kinds of copper (I) catalysts have been reported for CuAAC reactions. However, some of these catalysts have serious drawbacks. For instance, some of the reported catalysts suffer from their instability or high tendency toward oxidation, which leads to catalytic inactivity. In some cases, the formation of undesired alkyne–alkyne coupling and other by-products are observed in their presence [7,8]. Almost all of them are applicable and suitable for certain kinds of solvents. For example, simple coordination complexes of Cu(P(OMe)3)3Br [9] and Cu(PPh3)3Br [10,11] are often used in reactions in organic solvents, in which cuprous salts have limited solubility. A report describes the bis(phosphine) complex Cu(PPh3)2OAc as an excellent catalyst for the CuAAC reaction in toluene and dichloromethane [12]. In situ-prepared Cu (I) from Cu (II), using sodium ascorbate was introduced by Fokin et al. for click cyclization in water [6]. Although the immobilization of copper (I) salts on various supports makes them recoverable and somewhat more stable than their free copper (I) salt counterparts, the leaching of the catalysts from support into the reaction mixture leads to product contamination and catalyst deficiency during the recycling process.
Click chemistry principles are consistent with the goals of green chemistry, focusing on minimizing the hazard and maximizing the efficiency of any chemical choice. They have attracted an enormous amount of interest over the past decade [13].
Although CuAAC reactions can often be carried out in water as a green solvent, the solubility of organic azide and acetylene reactants can sometimes be a serious obstacle in using this reaction medium. In particular, a phase transfer catalyst or elevated temperatures seems to be necessary when organic azides are prepared in situ from their halide forms. Using ionic liquids as a reaction medium can overcome these problems. The user-friendly and adjustable properties of ionic liquids have prompted numerous applications as environmentally benign reaction media, catalysts [14], task-specific reagents [15], and chirality transfer media [16]. From this perspective, combining the synthetic potential of MCRs with the dual properties of room temperature ionic liquids (RTILs) as solvents and catalysts has resulted in the development of new and promising eco-compatible organic transformations [17]. Recently, a diversity of ionic liquids containing copper (I) and (II) have been synthesized and were used as catalysts for the oxidative carbonylation of alkanols to dialkyl carbonates [18,19]. This paper aims to introduce new application for the ionic liquid containing copper (I), [Cu(Im12)2]CuCl2, and demonstrates that the mixture of 1-butyl-3-methylimidazolium tetrafluoroborate ([bmim]BF4)/[Cu(Im12)2]CuCl2 is a versatile, recyclable catalytic reaction medium for multicomponent Huisgen preparation of 1,4-disubstituted 1,2,3-triazoles, adapted with the principles of green chemistry.
2 Experimental
2.1 Materials and methods
All α-halo ketones, ionic liquids and other chemicals were purchased from Fluka and Merck in high purity, and [Cu(Im12)2]CuCl2 was synthesized according to the literature [18]. All of the triazole compounds were prepared using our procedure. Their spectroscopic data were recorded on a BOMEM MB-Series 1998 FT-IR spectrophotometer, Bruker Avance DPX 500 MHz and DPX 400 MHz spectrometers using TMS as internal standard. Mass spectral analyses were made on an Agilent HP 5973 Network Mass Selective Detector. The analyzer made its determination according to the ASTM method. Elemental analyses were performed on a Thermo Fin-nigan CHNS-O analyzer, 1112 series.
2.2 Preparation of [Cu(Im12)2]CuCl2
To a Schlenk flask charged with CuCl (1.21 g, 12.22 mmol), a solution of 1-dodecylimidazole (3.06 g, 12.95 mmol) in CH3CN (5 mL) was added. It was placed in an ultrasonic bath at room temperature for 30 min. The solvent was evaporated in vacuo and the precipitate was washed with ether and dried under vacuum to give [Cu(Im12)2]CuCl2 as a fine crystalline colorless solid; anal. calc. for C30H56Cl2Cu2N4: C 53.72, H 8.41, N 8.35%; found: C 53.87, H 8.58, N 8.45%; mp 73 °C (Lit. [18] 73 °C), decomp. 281.4 °C. IR (cm−1): 3122, 3050, 2950, 2847, 1690, 1615, 1520, 1466, 1442, 1399, 1357, 1288, 1240, 1110, 1053, 1039, 1028, 1006, 962, 895, 845, 760, 730, 655, 500, 442.
2.3 General procedure for the synthesis of 1,4-disubstituted-1H-1,2,3-triazoles using [Cu(Im12)2]CuCl2 as a catalyst
[Cu(Im12)2]CuCl2 (0.07 g, 0.1 mmol) was added to a round-bottomed flask containing [bmim]BF4/H2O 1:1 (8 mL), α-halo ketone (1 mmol), terminal alkyne (1 mmol) and sodium azide (1.2 mmol). The reaction mixture was stirred at room temperature for 20 min. The organic phase was extracted with ethyl acetate (2 × 8 mL), washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The solid residual was recrystallized in hot ethanol to give pure crystals of the product. In some cases, water was added dropwise to precipitate the pure product (Table 3, entries 6–8). The aqueous phase was kept for the next runs. The pure products were dried under vacuum at room temperature, which resulted in 80–89% yields.
Synthesis of 1,4-disubstituted-1H-1,2,3-triazoles in the [bmim]BF4/H2O medium using Cu(I)-based catalytic ionic liquid.
| Entry | α-halo ketone(a) | Product(b) | Melting point | Yield (%)a |
| 1 | 146 | 89 | ||
| 2 | 145 | 86 | ||
| 3 | 142 | 81 | ||
| 4 | 152 | 84 | ||
| 5 | 138 | 84 | ||
| 6 | 112 | 84 | ||
| 7 | 108 | 83 | ||
| 8 | 118 | 81 | ||
| 9 | 146 | 82 | ||
| 10 | 163 | 82 | ||
| 11 | More than 300 | 80 |
a Yields refer to pure and isolated products.
2.4 Typical procedure for multicomponent synthesis of 2-(4-{4-[1-(2-oxo-2-phenylethyl)-1H-1,2,3-triazol-4-yl]phenyl)}-1H-1,2,3-triazol-1-yl-1-phenyl-1-ethanone
[Cu(Im12)2]CuCl2 (0.14 g, 0.2 mmol) was added to a round-bottomed flask containing [bmim]BF4/H2O 1:1 (16 mL), 1,3-diethynylbenzene (1 mmol), phenyl acetylene (2 mmol) and sodium azide (2.4 mmol). The reaction mixture was stirred at room temperature for 20 min (Table 3, entry 11b). The organic phase was extracted with ethyl acetate (2 × 16 mL), washed with brine, dried over anhydrous Na2SO4, filtered and concentrated in vacuo. The solid residual was recrystallized in hot ethanol to give pure crystals of the product. The latter was dried under vacuum at room temperature as a white solid crystal [20] (0.360 g, 80%). IR (cm−1):1696 (CO), 1226, 686; 1H-NMR (500 MHz DMSO-d6) δ 8.64 (2H, s, CH triazole), 8.44 (1H, s), 8.13–8.12 (4H, m), 7.87 (2H, q, J = 2.33 Hz,), 7.76 (2H, t, J = 4.95 Hz), 7.64 (4H, t, J = 5.17 Hz,), 7.58 (1H, t, J = 5.17 Hz), 6.30 (4H, s, COCH2); 13C-NMR (125 MHz DMSO-d6) δ 193.0, 146.9, 135.1, 135.0, 132.2, 130.5, 129.9, 129.1, 125.5, 122.7, 56.9; anal. calcd. for C28H20N6O2: C 69.63, H 4.49, N 18.74%, found: C 69.22, H 5.56, N 18.81.
1-phenyl-2-(4-phenyl-1H-1,2,3-triazole-1-yl)-1-ethanon (1b of Table 3, 89%, white solid crystals) [21]: IR (cm−1): 1704 (CO), 1H-NMR(400 MHz, DMSO-d6): δ 8.54 (1 H, s), 8.11 (2H, d; J = 8.10 Hz), 7.90–7.88 (2H, m), 7.78–7.74 (1H, m), 7.63 (2H, t; J = 7.7 Hz), 7.47(2 H, t; J = 7.7 Hz), 7.38–7.34 (1H, m), 6.28(2H, s). 13C-NMR (100 MHz, DMSO-d6): δ 192.7 (CO), 146.8, 134.8, 134.6, 131.2, 129.5, 129.4, 128.7, 128.4, 125.6, 123.5, 56.5. HRMS (CI): MH+, C12H12BrN3O2 requires 263.2939, found: 263.3413.
1-(4-phenyl-1H-1,2,3-triazole-1-yl)acetone (5b of Table 3, white solid crystals). IR (cm−1): 1710 (CO), 1H-NMR(500 MHz, CDCl3): δ 7.88–7.87(3H, m), 7.46(2H, t, J = 7.50 Hz), 7.40–7.36(1H, m), 5.28(2H, s), 2.30(3H, s). 13C-NMR(125 MHz, CDCl3): δ 199.61 (CO), 148.64, 130.77, 129.30, 128.74, 126.22, 121.55, 58.96, 27.66. HRMS (CI): MH+, C12H12BrN3O2 requires 201.2, found: 201.2.
1-(4-bromophenyl)-2-[(4-(1-hydroxyethyl)-1H-1,2,3-triazole-1-yl)]-1-ethanone (7b of Table 3, 0.191 g, 83%, white solid crystals) [21]. IR (cm−1): 3417 (OH), 1704 (CO), 1599, 1497, 1250 cm−1; 1H-NMR (400 MHz DMSO-d6) δ 8.37 (1H, s, CH triazole), 7.95 (2H, d, J = 8.6 Hz), 7.79 (2H, d, J = 8.60 Hz,), 5.96 (2H, s, COCH2), 4.95 (1H, s, OH), 4.19–4.16 (1H, m), 1.54 (3H, d, J = 5.20 Hz, CH3); 13C-NMR (125 MHz CDCl3) δ 189.82, 153.86, 148.81, 132.67, 130.86, 130.48, 121.47, 52.37, 45.97, 28.13; HRMS (CI): MH+, C12H12BrN3O2 requires 310.2, found: 310.2; anal. calcd. for C12H12BrN3O2: C 46.47, H 3.90, N 13.55%; found: C 46.34, H 3.91, N 13.34.
2-[4-(1-hydroxyethyl)-1H-1,2,3-triazole-1-yl]-1-(4-methoxyphenyl)-1-ethanone (8b of Table 3, 0.212 g, 81%, white solid crystals). IR (cm−1): 3503 (OH), 1694 (CO), 1597, 1232, 1170 cm−1; 1H-NMR (500 MHz CDCl3) δ 8.06 (2H, d, J = 8.74 Hz,), 7.87 (1H, s, CH triazole), 7.13 (2H, d, J = 8.75 Hz), 6.07 (2H, s, COCH2), 5.28 (1H, s), 4.89–4.86 (1H, m), 3.89 (3H, s), 1.44 (3H, d, J = 8.75 Hz); 13C-NMR (125 MHz CDCl3) δ 189.82, 153.86, 148.81, 132.67, 130.86, 130.48, 121.47, 52.37, 45.97, 28.13; anal. calcd. for C13H15N3O3: C 59.76, H 5.79, N 16.08%; found: C 60.11, H 5.65, N 16.25; HRMS (CI): MH+, C13H15N3O3 requires 261.3, found: 260.3.
2.5 Reusing of the reaction medium
The aqueous phase from Section 2.3 (using phenacyl bromide and phenyl acetylene) was used for synthesis of 1-phenyl-2-(4-phenyl-1H-1,2,3-triazol-1-yl)ethanone by adding phenacyl bromide (1 mmol), phenyl acetylene (1 mmol) and sodium azide (1.2 mmol) in the next similar runs.
3 Results and discussion
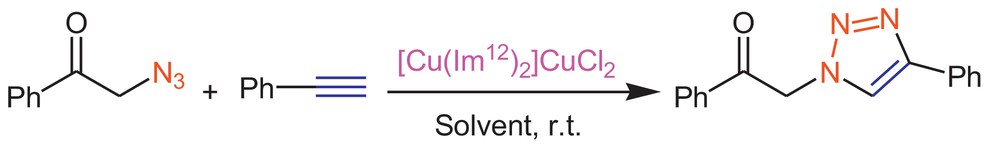
An ionic liquid containing copper (I), [Cu(Im12)2]CuCl2, was prepared according to the reported method [18]. To evaluate the efficiency of this ionic liquid as the catalyst for the azide–alkyne cycloaddition reaction in various solvents, click cyclization between phenacyl azide and phenyl acetylene to afford 1-phenyl-2-(4-phenyl-1H-1,2,3-triazole-1-yl)-1-ethanone (1b) was selected as a model reaction (Scheme 1).
Evaluation of ionic liquid containing copper (I) for click cyclization in various solvents.
The reaction progress was studied in [bmim]BF4, H2O, [bmim]BF4/H2O, THF/H2O, DMF/H2O, dioxane/H2O and CH2Cl2 under neat conditions. The results of using this task-specific ionic liquid in various solvents are reported (Table 1). The IR spectra of Fig. 1b exhibited a strong band at about 1702 cm−1, indicating the presence of a ketonic functionality. Its 1H-NMR showed a characteristic singlet at δ 8.53 for triazolyl C5–H. High-resolution mass spectra of the title compound exhibited the molecular ion peak at m/z 263.34, which is in agreement with the calculated value, m/z 263.29.
Evaluation of the efficiency of [Cu(Im12)2]CuCl2 for click reaction between phenacyl azide and phenyl acetylene in various solvents.
| Entry | Solvent | Time (min) | Yield (%)a |
| 1 | Neat | 25 | 87 |
| 2 | [bmim]BF4 | 10 | 89 |
| 3 | DMF/H2O 1:1 | 60 | 73 |
| 4 | CH2Cl2 | 60 | 75 |
| 5 | [bmim](BF4/H2O 1:1) | 15 | 90 |
| 6 | CH3CN | 60 | 83 |
| 7 | THF | 60 | 77 |
| 8 | THF/H2O 1:1 | 60 | 82 |
a Isolated yield.
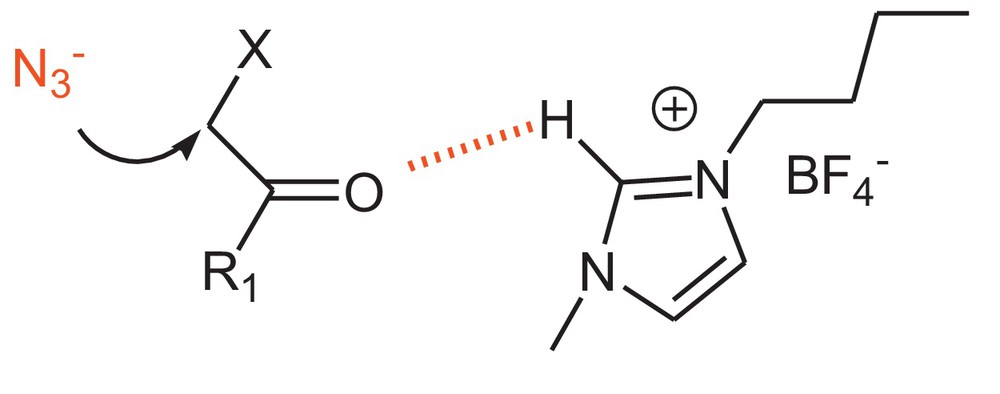
Acidic hydrogen of imidazolium cation promotes the nucleophilic substitution at the α-position of α-halo ketones.
Although some efforts have been made on the use of ionic liquids as green solvents for CuAAC reaction, these methods have long reaction times or require additive for effective click cyclization [22–24]. These reports do not represent the catalytic activity of ionic liquids and are used only as a suitable medium to increase the solubility of the reactants.
It is clear that the task-specific ionic liquid, [Cu(Im12)2]CuCl2, works very well as a catalyst in various solvents even under neat condition and reduces the reaction times compared with the reported catalysts. This catalytic ionic liquid is the first type of ionic liquids that can catalyze click cyclization. Encouraged by these successful results, we decided to design a green methodology for the synthesis of 1,4-disubstituted 1,2,3-triazoles directly from α-halo ketones using task-specific ionic liquids (TSILs) as the reaction medium and catalyst. To the best of our knowledge, there is no extant report describing this methodology. We first embarked on the evaluation of RTILs and the comparison of their efficiency in the MCR. Multicomponent click cyclization between phenacyl bromide and phenyl acetylene was selected as the model reaction and its behavior was investigated using a diversity of ionic liquids as solvents (Scheme 2 and Table 2).

Evaluation of various solvents for the multicomponent reaction catalyzed by ionic liquid containing copper (I).
The effect of ionic liquids as solvent for multicomponent click cyclization.
| Entry | Solvent | Time (min) | Yield (%)a |
| 1 | [bmim]BF4 | 60 | 78 |
| 2 | [bmim]PF6 | 60 | 62 |
| 3 | [hmim]BF4 | 60 | 73 |
| 4 | [bmmim]BF4 | 60 | 65 |
| 5 | [bmim]BF4/H2O 1:1 | 20 | 89 |
| 6 | [bmim]BF4/H2O 1:3 | 20 | 81 |
a Yields refer to isolated and pure products.
Looking at the anions of ionic liquid, BF4–produced a higher yield than PF6−(Table 2, entries 1, 2). The imidazolium cation has a critical role in the first step of the reaction (Scheme 3). The formation of a hydrogen bond between the acidic C2 hydrogen of imidazolium cation [25] and the carbonyl group of phenacyl halide activates this substrate (Fig. 1 and Table 2, entries 1 and 3). The absence of this proton decreases the reactivity (Table 2, entry 4).
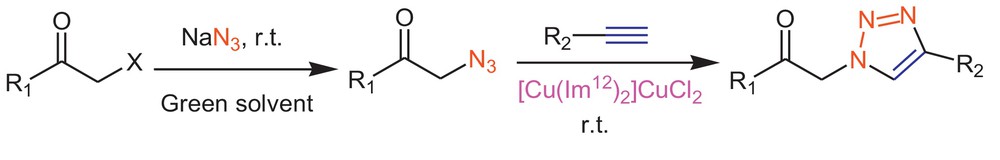
Stepwise synthesis of 1,4-disubstituted-1H-1,2,3-triazoles.
In addition, the branch of imidazolium cation has affected the reaction yields (Table 1, entries 1 and 3). The acidity of ionic liquids with BF4−anion can be finely tuned via cation variation, whereas ionic liquids with PF6−are more stable and result in neutral aqueous solutions [26].
From Table 2 and the discussion above, the mixture of [bmim]BF4/H2O (1:1 v/v) stands out as the solvent of preference with its fast reaction rate and high yield of the product. Water, as a co-solvent, makes sodium azide to be soluble during nucleophilic substitution reaction. Moreover, water increases the acidity of [bmim]BF4 [26] (Table 2, entries 5 and 6), because BF4− is hydrolyzed into H+ and HOBF3− in this medium [27–30]. These effects have a direct relationship with the observed rate of the reaction. Consequently, in order to exhibit the efficiency of [bmim]BF4/H2O as a solvent for a wide range of substrates, multicomponent click cyclization reaction between various types of α-halo ketones and terminal alkynes was explored in [bmim]BF4/H2O (1:1 v/v) using [Cu(Im12)2]CuCl2 as a catalyst. The results of using TSIL, [Cu(Im12)2]CuCl2, as a catalyst and [bmim]BF4/H2O as a solvent are reported (Scheme 4 and Table 3). Structural assignments of the products were confirmed by the appearance of a singlet in the 7.8–8.5 ppm range in 1H-NMR spectra and approved the regioselective synthesis of 1,4-disubstituted triazole regioisomers.
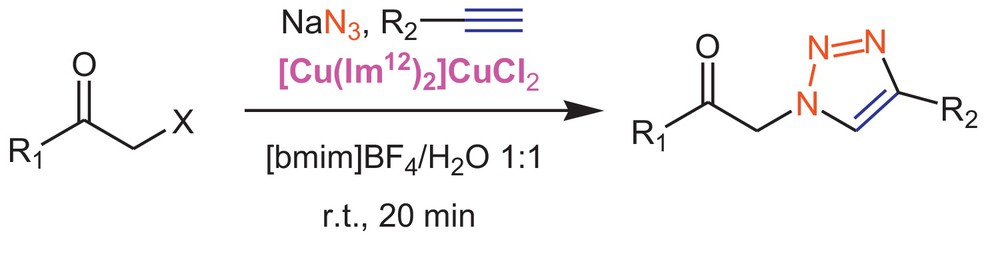
Multicomponent synthesis of 1,4-disubstituted-1H-1,2,3-triazoles in the [bmim]BF4/H2O medium using Cu(I)-based ionic liquid catalyst.
In all cases, multicomponent click cyclization was completed in excellent time and the 1,2,3-triazole derivatives were isolated with high yields. These observations clearly indicate the generality and scope of the reaction with respect to various terminal alkynes and α-halo ketones.
A true and efficient catalyst can be simply recovered and reused. To investigate these properties for our introduced catalyst, the reaction of phenacyl bromide and phenyl acetylene was selected again as a model reaction. After completion of click cyclization, the product was easily extracted from ionic liquid solvent and catalyst using EtOAc. The recovered ionic liquid mixture was reused in the next run. Almost consistent activity was observed using [bmim]BF4/H2O as solvent and [Cu(Im12)2]CuCl2 as a catalyst over five runs (Fig. 2).

Recyclability and reusability study of the reaction medium.
The yield difference between the first and fifth runs was only 4%. These observations suggest that the efficiency of the introduced catalytic ionic liquid medium is maintained through repeated uses. The copper contents of the fresh and the reused catalyst (after the fifth run) were measured using the ICP–AES technique. In both cases, the results were identical and showed that the title catalyst was almost perfectly extracted into the aqueous phase.
In order to compare the efficiency of the ionic liquid containing the copper (I) catalyst with some of the traditional copper (I) catalysts, the CuAAC reaction between phenacyl azid and phenyl acetylene was investigated using these catalysts in [bmim]BF4/H2O (Table 4). Although using copper (I) salts leads to the synthesis of 1,4-disubstituted-1H-1,2,3-triazoles, they do not give good yields, have long reaction times and suffer from the difficulty of separation and reusability (Table 4, entries 1–3). Another deficiency of such catalysts is that they have not been recommended for aqueous mediums [31]. Although in situ-prepared Cu(I) forms CuSO4, sodium ascorbate is a suitable catalyst for aqueous medium and gives high yields, this catalyst needs relatively long reaction times and is not recyclable (Entry 4). [Cu(Im12)2]CuCl2 is a greener catalyst; thermodynamically more stable (its thermal decomposition temperature is 234 °C) [18], gives the product in shorter times at higher yields and can be simply recovered.
Comparison of the efficiency of the copper-containing ionic liquid with some of the traditional catalysts for the CuAAC reaction.
| Entry | Catalyst | Time (min) | Yield (%)a,b |
| 1 | CuCl | 90 | 58 |
| 2 | CuCN | 90 | 65 |
| 3 | CuBr | 90 | 68 |
| 4 | CuSO4/NaAsc | 90 | 87 |
| 5 | [Cu(Im12)2]CuCl2 | 20 | 89 |
a All reactions were carried out using 0.5 mmol phenacyl bromide, 0.5 mmol phenyl acetylene, copper(I) catalyst (10 mol%), 0.6 mmol NaN3 in [bmim]BF4/H2O (4 mL 1:1) at room temperature.
b Isolated yields.
The mechanistic pathway mediated by [Cu(Im12)2]CuCl2 is depicted in Fig. 3.

Proposed mechanism for multicomponent synthesis of 1,4-disubstituted-1H-1,2,3-triazoles using task-specific ionic liquids as solvent and catalyst.
4 Conclusions
A new and green methodology is introduced for one-pot multicomponent synthesis of 1,4-disubstituted-1H-1,2,3-triazoles from α-halo ketones usage of ionic liquids as solvent and catalyst for this reaction. Obtaining of high yields in short times, use of a recoverable and reusable task-specific copper-containing ionic liquid as a catalyst, and its cost effective and eco-friendly properties make this method interesting and adjustable with sustainable chemistry.
Acknowledgment
The authors gratefully acknowledge the Research Council of Yasouj University for financial support.


