1 Introduction
Multicomponent reaction (MCR) is a one-pot reaction in which three or more reactants are combined together to form a new desired compound without isolation of any intermediate [1]. MCR, designed to produce biologically active compounds, has become an important area of research in organic, combinatorial and medicinal chemistry [2].
Tandem reaction is the combination of two or more reactions whose occurrence is in a specific order [3]. These reactions are also one-pot multi-step processes and hence, very powerful for the rapid construction of complex organic molecules in a facile and efficient manner [3]. These reactions fall under the fold of green chemistry, as they obviate the isolation and purification of intermediates leading to diminished pollution of the environment [4].
Tandem Knoevenagel–Michael reaction is known in classical organic chemistry [3], and until now the investigations are under progress [5–12].
Sodium acetate is an inexpensive, non-toxic and readily available catalyst for some organic reactions. It was used as a weakly base catalyst for aldol condensation of aromatic aldehydes and acid anhydrides (Perkin reaction) [13], for Knoevenagel condensation of carbonyl compounds [14] and for the condensation of hippuric acid with aromatic aldehydes (Erlenmeyer–Plöchl reaction) [15]. We have found sodium acetate to catalyze multicomponent cyclization of aryl aldehydes, malononitrile and acetone into cis-4-dicyanomethylene-2,6-diarylcyclohexane-1,1-dicarbonitrile [16]. Recently, sodium acetate was used as the catalyst for MCR of diethyl but-2-enedioate, malononitrile, formaldehyde and aromatic amine to form multisubstituted 1,2,3,4-tetrahydropiridines [17].
Functionally substituted 2-pyrazolin-5-ones have received considerable attention in the field of medicinal chemistry [18,19]. Thus, the N-methyl derivative of 3-methyl-1-phenyl-2-pyrazolin-5-one represents the first truly synthetic pain reliever antipyrine, which is an approved non-steroidal anti-inflammatory drug (NSAID), possessing analgesic and antipyretic activities [20]. Different types of 4-substituted 3-methyl-2-pyrazolin-5-ones (or their hydroxy tautomers) have been reported as anticonvulsant [21], antidiabetic [22], neuroleptic [23], antihyperlipidemic [24], and gastric secretion stimulatory agents [25], as well as multidrug resistance modulators for cancer and antimicrobial therapy [26]. The current interest in 4-substituted 3-methyl-2-pyrazolin-5-one derivatives bearing nitrile functionality, especially substituted 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles, arises from their potential application in the treatment of cardiovascular diseases through the dual inhibition of phosphodiesterase-1 and 5 in blood vessels [27].
The most efficient conventional approach to the synthesis of corresponding functionalized 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles utilizes the Michael addition of 3-methyl-2-pyrazolin-5-ones to the electron-deficient arylidenemalononitriles under neutral reaction conditions in an alcoholic solvent [28–30]. Although this process leads to the formation of the desired products in 70–80% yields over 1–4 h reaction period, it requires preliminary preparation of arylidenemalononitrile that constitutes an additional separate synthetic stage (Knoevenagel reaction). According to known literature protocols, neutral reaction conditions are the essential requirement to obtain the corresponding 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles in a selective manner with a preparative yield as the rational catalysis of 3-methyl-2-pyrazolin-5-ones and arylidenemalononitriles with a base activates a rapid intramolecular cyclization of the desired Michael adducts into the corresponding 6-amino-1,4-dihydropyrano[2,3-c]pyrazoles [29,30]. An analogous reaction result was also observed in the case of three-component condensation of 3-methyl-2-pyrazolin-5-ones, aryl aldehydes and malononitrile in the presence of a base [28].
Recently, we have accomplished the multicomponent one-step synthesis of functionalized 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles as the electrocatalytic multicomponent transformation of aryl aldehydes, 2-pyrazolin-5-ones and cyano-functionalized C–H acids into substituted 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles [31]. This electrochemically induced process is the classical tandem Knoevenagel–Michael reaction, which occurs under very weak base conditions near the surface of the cathode during the electrolysis in an undivided cell without any further cyclization step [31].
Considering our results on the electrocatalytic chain transformation of aryl aldehydes, 2-pyrazolin-5-ones and C–H acids as well as certain biomedical application of 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles mentioned above, we were prompted to design a convenient and facile catalytic MCR methodology for the efficient multicomponent synthesis of the functionalized 4-substituted 3-methyl-2-pyrazolin-5-one system based on the usage of sodium acetate as a weak base catalyst in the chain MCR of aldehydes, 3-methyl-2-pyrazolin-5-ones and cyano-functionalized C–H acids.
2 Results and discussion
As it follows from the introduction, we were prompted to design a convenient and facile catalytic MCR methodology for the efficient multicomponent synthesis of functionalized 4-substituted 3-methyl-2-pyrazolin-5-one system. Thus, in the present study, we report our results on the study of sodium acetate as a weak base catalyzed multicomponent chain transformation of aryl aldehydes 1a–e, 3-methyl-2-pyrazolin-5-ones 2a,b and cyano-functionalized C–H acids 3a–c into substituted 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles 4a–k under mild conditions in alcohols (Scheme 1, Table 1).
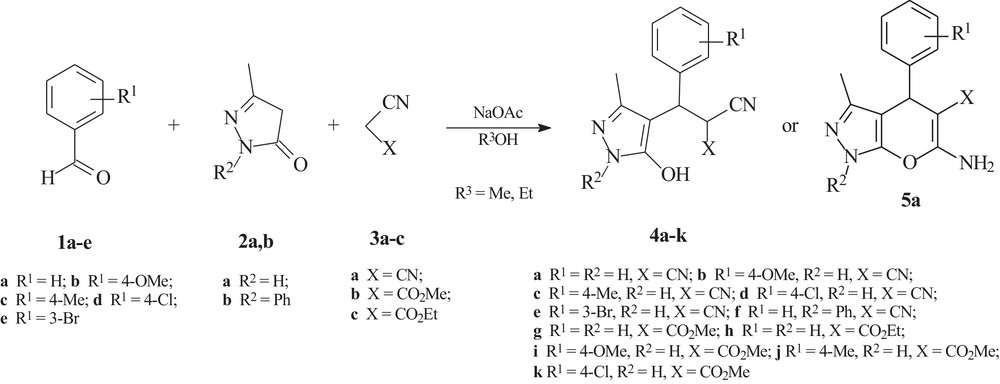
Sodium acetate catalyzed multicomponent transformation of aryl aldehydes, 3-methyl- 2-pyrazolin-5-ones and cyano-functionalized C–H acids.
Multicomponent condensation of aryl aldehydes 1, 2-pyrazolin-5-ones 2 and C–H acids 3 into 3-(5-hydroxypyrazol-4-yl)-3-arylpropionitriles 4a–ka.
| Entry | Aldehyde | Pyrazolin-5-one | C–H acid | Alcohol | Reaction time (min) | Product | Yield (%)b |
| 1 | 1a | 2a | 3a | EtOH | 30 | 4a | 83 |
| 2 | 1a | 2a | 3a | EtOH | 60 | 5a | 86 |
| 3 | 1b | 2a | 3a | EtOH | 30 | 4b | 78 |
| 4 | 1b | 2a | 3a | EtOH | 60 | 4b | 92 |
| 5 | 1c | 2a | 3a | EtOH | 30 | 4c | 81 |
| 6 | 1c | 2a | 3a | EtOH | 60 | 4c | 94 |
| 7 | 1d | 2a | 3a | EtOH | 30 | 4d | 85 |
| 8 | 1d | 2a | 3a | EtOH | 60 | 4d | 97 |
| 9 | 1e | 2a | 3a | EtOH | 60 | 4e | 99 |
| 10 | 1a | 2b | 3a | EtOH | 30 | 4f | 83 |
| 11 | 1a | 2a | 3b | MeOH | 60 | 4g | 90 |
| 12 | 1a | 2a | 3c | EtOH | 60 | 4h | 91 |
| 13 | 1b | 2a | 3b | MeOH | 60 | 4i | 91 |
| 14 | 1c | 2a | 3b | MeOH | 60 | 4j | 88 |
| 15 | 1d | 2a | 3b | MeOH | 60 | 4k | 86 |
a 10 mmol of aldehyde 1, 10 mmol of pyrazolin-5-one 2, 10 mmol of C–H acid 3, 1 mmol of NaOAc, 20 mL of alcohol, 20 °C.
b Yield of isolated product (isolated by filtration of reaction mixture).
Excellent conversions of the starting compounds were obtained under all the conditions studied. In the main part of the experiments (Table 1, entries 1,3–15), the only 3-(5-hydroxypyrazol-4-yl)-3-arylpropionitriles 4a–k were isolated in the NaOAc-catalyzed reaction in alcohols at 20 °C. Also, in the main part of the experiments (entries 4,6,8,9,11-15, Table 1), 1 h reaction time is needed to obtain the best yields of 3-(5-hydroxypyrazol-4-yl)-3-arylpropionitriles 4b–k; under these conditions 4b-k were isolated in 86–99% yields. The only exception was in the case of NaOAc-catalyzed MCR of benzaldehyde 1a, 3-methyl-2-pyrazolin-5-one 2a and malononitrile 3a in methanol (Table 1, entries 1 and 2). In this case 3-(5-hydroxypyrazol-4-yl)-3-arylpropionitrile 4a was isolated in 30 min in 83% yield, but in 1 h under the same conditions cyclic 6-amino-3-methyl-4-phenyl-1,4-dihydro[2,3-c]pyrazole-5-carbonitrile 5a was obtained in 86% yield.
Nevertheless, in a period of 30 min, 3-(5-hydroxypyrazol-4-yl)-3-arylpropionitriles 4b–d,f were also obtained in high 78–85% yield (Table 1, entries 3,5,7,10).
Two special experiments were carried out to check the mechanism of the catalytic MCR studied: the NaOAc-catalyzed reaction of aldehyde 1b with malononitrile 3 in methanol at 20 °C and the NaOAc-catalyzed reaction of aldehyde 1b with 3-methyl-2-pyrazolin-5-ones 2a in methanol at 20 °C. In the reaction of aldehyde 1b with malononitrile 3a under these conditions, (4-methoxybenzylidene)malononitrile 6 was obtained in 98% yield; the analogous reaction of aldehyde 1b with 3-methyl-2-pyrazolin-5-ones 2a resulted in only 33% conversion of aldehyde 1b with the formation of multiple condensation products.
With the above results taken into consideration and the mechanistic data on the sodium acetate catalyzed multicomponent cyclization of aryl aldehydes, malononitrile and acetone into cis-4-dicyanomethylene-2,6-diarylcyclohexane-1,1-dicarbonitrile previously performed by us [16], the following mechanism for the sodium acetate catalyzed multicomponent transformation of aldehydes 1, 3-methyl-2-pyrazolin-5-ones 2 and cyano-functionalized C–H acids 3 into substituted 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles 4 is proposed. The initiation step of the catalytic cycle begins with the deprotonation of a molecule of cyano-functionalized C–H acid 3 by the action of sodium acetate, which leads to the anion of cyano-functionalized C–H acid A formation (Scheme 2).
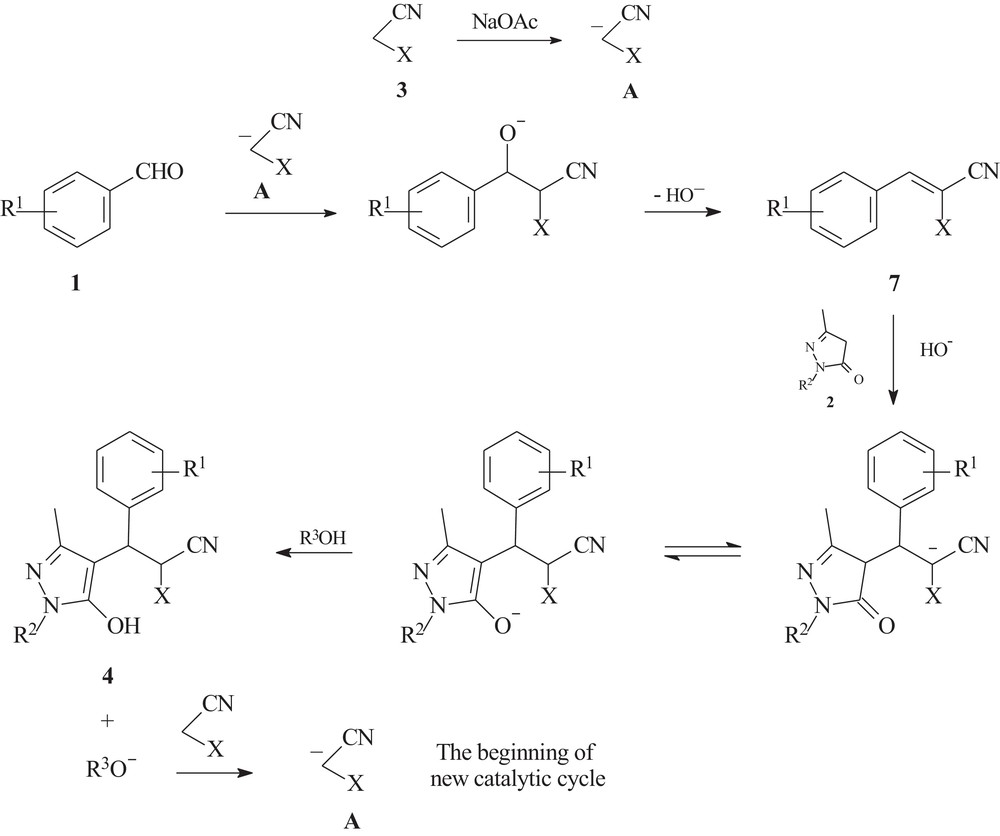
Mechanism of multicomponent transformation of aryl aldehydes, 3-methyl-2-pyrazolin-5-ones and cyano-functionalized C–H acids.
The following process in the solution represents a typical tandem reaction. Knoevenagel condensation of the anion A with aryl aldehyde 1 takes place with the elimination of a hydroxide anion and the formation of the Knoevenagel adduct 7 [33]. The subsequent hydroxide-promoted Michael addition of 3-methyl-2-pyrazolin-5-one 2 to the electron-deficient Knoevenagel adduct 7 leads to the corresponding 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitrile 4 with the regeneration of the alkoxide anion as the last step. The catalytic chain process then continues by the interaction of the alkoxide with the next molecule of C–H acid 3 (Scheme 2).
Thus, under the conditions of the sodium acetate catalyzed process, the generation of even a single anion of cyano-functionalized C–H acid 3 is theoretically sufficient for the total conversion of equimolar quantities of aldehyde 1, 3-methyl-2-pyrazolin-5-one 2, and cyano-functionalized C–H acid 3 into the corresponding 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitrile 4.
3 Conclusions
Thus, sodium acetate as the catalyst can produce, under mild conditions, a fast and selective tandem Knoevenagel–Michael reaction of aryl aldehydes, 3-methyl-2-pyrazolin-5-ones, and cyano-functionalized C–H acids to give 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles in excellent yields. The new catalytic chain process opens an efficient and convenient sodium acetate catalyzed multicomponent way to create corresponding 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles – the promising compounds for human cardiovascular diseases therapy and different biomedical applications. This is also the first example of the base catalyst induced tandem Knoevenagel–Michael reaction, which proceeds as the classical tandem Knoevenagel–Michael reaction without any further cyclization. The catalytic procedure utilizes a simple equipment; it is easily carried out and is valuable from the viewpoint of environmentally benign diversity-oriented large-scale processes. This efficient sodium acetate catalyzed approach to corresponding 3-(5-hydroxy-3-methylpyrazol-4-yl)-3-arylpropionitriles represents a new synthetic concept for MCRs, and allows combination of the synthetic virtues of conventional MCR with ecological benefits and convenience of sodium acetate catalyzed procedure.
4 Experimental
4.1 General remarks
All melting points were measured with a Gallenkamp melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded in DMSO-d6 and CDCl3 with a Bruker Avance II 300 spectrometer at ambient temperature. Chemical shift values are relative to Me4Si. IR spectra were recorded with a Bruker ALPHA-T FT-IR spectrometer in KBr pellets. Mass-spectra (EI = 70 eV) were obtained directly with a Kratos MS-30 spectrometer. All the chemicals used in this study were commercially available.
4.2 General procedure
A solution of aryl aldehyde (10 mmol), 2-pyrazolin-5-one (10 mmol), cyano-functionalized C–H acid (10 mmol) and sodium acetate (0.082 g, 1 mmol) in MeOH or EtOH alcoholic solvent (20 mL) was stirred in a flask equipped with a magnetic stirrer at 20 °C for 1 h. After the reaction was finished, the solution was filtered out to isolate the solid product, which was then rinsed with an ice-cold alcohol/water solution (9:1, 3 mL), and dried under reduced pressure.
4.2.1 [(5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(phenyl)methyl]malononitrile (4a)
White solid (2.09 g, yield 83%); mp 256–258 °C (lit [28] mp 258–259 °C); 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.08 (s, 3H, CH3), 4.63 (d, J = 11.4 Hz, 1H, CH), 5.52 (d, J = 11.4 Hz, 1H, CH), 7.24–7.37 (m, 3H, Ar), 7.47–7.49 (m, 2H, Ar), 10.72 (br s, 1H).
4.2.2 [(5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-methoxyphenyl)methyl]malononitrile (4b)
White solid (2.59 g, yield 92%); mp 207–208°С; 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.07 (s, 3H, CH3), 3.73 (s, 3H, OCH3), 4.57 (d, J = 11.4 Hz, 1H, CH), 5.45 (d, J = 11.4 Hz, 1H, CH), 6.91 (d, J = 8.4 Hz, 2H, Ar), 7.41 (d, J = 8.4 Hz, 2H, Ar), 10.85 (br s, 1H); 13C NMR (75 MHz, DMSO-d6): δ (ppm) 9.7, 18.5, 27.7, 40.8, 55.0, 98.9, 113.9 (2 C), 114.1, 128.9 (2 C), 131.8, 137.8, 158.6, 159.0; MS (m/z, relative intensity %): 282 ([M]+, 28), 256 (3), 221 (7), 216 (100), 184 (58), 175 (84), 159 (22), 115 (39), 109 (50), 98 (28); IR (KBr): ν = 3484, 3358, 3257, 2962, 2192, 1644, 1512, 1392, 1260, 1172 cm−1; Anal calcd for C15H14N4O2 (%): C, 63.82; H, 5.00; N, 19.85; Found (%): C, 63.69; H, 5.15; N, 19.71.
4.2.3 [(5-Hydroxy-3-methyl-1H-pyrazol-4-yl)(4-methylphenyl)methyl]malononitrile (4c)
White solid (2.50 g, yield 94%); mp 207–209 °C (lit [28] mp 208–209 °C); 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.08 (s, 3H, CH3), 2.27 (s, 3H, CH3), 4.58 (d, J = 11.3 Hz, 1H, CH), 5.49 (d, J = 11.3 Hz, 1H, CH), 7.15 (d, J = 8.0 Hz, 2H, Ar), 7.37 (d, J = 8.0 Hz, 2H, Ar), 11.04 (br s, 1H).
4.2.4 [(4-Chlorophenyl)(5-hydroxy-3-methyl-1H-pyrazol-4-yl)methyl]malononitrile (4d)
White solid (2.77 g, yield 97%); mp 229–230 °C (lit [31] mp 229–230 °C); 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.10 (s, 3H, CH3), 4.70 (d, J = 11.3 Hz, 1H, CH), 5.53 (d, J = 11.3 Hz, 1H, CH), 7.43 (d, J = 8.5 Hz, 2H, Ar), 7.52 (d, J = 8.5 Hz, 2H, Ar), 10.98 (br s, 1H).
4.2.5 [(3-Bromophenyl)(5-hydroxy-3-methyl-1H-pyrazol-4-yl)methyl]malononitrile (4e)
White solid (3.26 g, yield 99%); mp 216–217 °C (lit [31] mp 216–217 °C); 1H NMR (300 MHz, DMSO-d6): δ (ppm) 2.11 (s, 3H, CH3), 4.70 (d, J = 11.4 Hz, 1H, CH), 5.57 (d, J = 11.4 Hz, 1H, CH), 7.33 (t, J = 7.8 Hz, 1H, Ar), 7.46–7.54 (m, 2H, Ar), 7.74 (s, 1H, Ar), 11.03 (br s, 1 H).
4.2.6 [(5-Hydroxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)(phenyl)methyl]malononitrile (4f)
White solid (2.72 g, yield 83%); mp 171–172 °C (lit [29] mp 173–174 °C); 1H NMR (300 MHz, DMSO-d6): 2.20 (s, 3H, CH3), 4.73 (d, J = 10.8 Hz, 1H, CH), 5.92 (d, J = 10.8 Hz, 1H, CH), 7.15–7.74 (m, 10H, Ar), 11.60 (br s, 1H).
4.2.7 Methyl 2-cyano-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-3-phenylpropanoate (4g)
White solid, mixture of two diastereoisomers (1:1), (2.57 g, yield 90%); mp 172–173 °C (lit [31] mp 172–173 °C); 1H NMR (300 MHz, DMSO-d6, double set of signals): δ (ppm) 1.98 (s, 3H, CH3), 2.06 (s, 3H, CH3), 3.52 (s, 3H, OCH3), 3.64 (s, 3H, OCH3), 4.35–4.41 (m, 2H, 2CH), 4.79–4.84 (m, 2H, 2CH), 7.19–7.39 (m, 8H, Ar), 7.48–7.51 (m, 2H, Ar), 10.75 (br s, 2H).
4.2.8 Ethyl 2-cyano-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-3-phenylpropanoate (4h)
White solid, mixture of two diastereoisomers (1:1), (2.72 g, yield 91%); mp 182–183 °C (lit [3] mp 182–183 °C); 1H NMR (300 MHz, DMSO-d6, double set of signals): δ (ppm) 0.88 (t, J = 7.1 Hz, 3H, CH3), 1.00 (t, J = 7.1 Hz, 3H, CH3), 1.98 (s, 3H, CH3), 2.05 (s, 3H, CH3), 3.88–3.96 (m, 2H, OCH2), 4.00–4.12 (m, 2H, OCH2), 4.29–4.37 (m, 2H, 2CH), 4.75–4.79 (m, 2H, 2CH), 7.15–7.38 (m, 8H, Ar), 7.48–7.51 (m, 2H, Ar), 10.95 (br s, 2H).
4.2.9 Methyl 2-cyano-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-3-(4-methoxyphenyl)propanoate (4i)
Yellowish solid, mixture of two diastereoisomers (1:1), (2.87 g, yield 91%); mp 159–160 °C; 1H NMR (300 MHz, DMSO-d6, double set of signals): δ (ppm) 1.97 (s, 3H, CH3), 2.05 (s, 3H, CH3), 3.53 (s, 3H, OCH3), 3.62 (s, 3H, OCH3), 3.70 (s, 3H, OCH3), 3.72 (s, 3H, OCH3), 4.29–4.36 (m, 2H, 2CH), 4.72–4.79 (m, 2H, 2CH), 6.83–6.90 (m, 4H, Ar), 7.27–7.30 (m, 2H, Ar), 7.40–7.43 (m, 2H, Ar), 10.78 (br s, 2H); 13C NMR (75 MHz, DMSO-d6, double set of signals): δ (ppm) 9.9, 10.0, 40.3, 40.6, 41.0, 41.1, 41.6, 53.0, 53.2, 55.0 (2C), 99.5, 99.9, 113.8 (4C), 116.9, 128.6 (2C), 129.0 (2C), 132.5, 132.6, 137.3, 137.5, 158.2, 158.3, 159.1, 159.2, 166.0, 166.1; MS (m/z, relative intensity %): 315 ([M]+, 8), 283 (3), 218 (9), 216 (100), 208 (5), 186 (24), 159 (19), 109 (16), 68 (43), 59 (48); IR (KBr): ν = 3474, 3397, 2960, 2240, 1747, 1609, 1516, 1306, 1030, 809 cm−1; Anal calcd for C16H17N3O4 (%): C, 60.94; H, 5.43; N, 13.33; Found (%): C, 60.72; H, 5.52; N, 13.20.
4.2.10 Methyl 2-cyano-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-3-(4-methylphenyl)propanoate (4j)
White solid, mixture of two diastereoisomers (1:1), (2.63 g, yield 88%), mp 180–181 °C; 1H NMR (300 MHz, DMSO-d6, double set of signals): δ (ppm) 1.94 (s, 3H, CH3), 2.02 (s, 3H, CH3), 2.23 (s, 3H, CH3), 2.26 (s, 3H, CH3), 3.52 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 4.28–4.34 (m, 2H, 2CH), 4.74–4.79 (m, 2H, 2CH), 7.07–7.14 (m, 4H, Ar), 7.21–7.24 (m, 2H, Ar), 7.34–7.36 (m, 2H, Ar), 10.88 (br s, 2H); 13C NMR (75 MHz, DMSO-d6, double set of signals): δ (ppm) 9.9, 10.0, 20.6 (2C), 40.7, 41.0, 41.4 (2C), 53.0, 53.2, 99.4, 99.8, 116.8 (2C), 127.3 (2C), 127.7 (2C), 129.0 (4C), 136.2 (2C), 137.3, 137.4, 137.5 (2 C), 159.1, 159.3, 165.9, 166.0; MS (m/z, relative intensity %): 299 ([M]+, 7), 267 (6), 208 (5), 202 (5), 201 (49), 185 (28), 180 (100), 128 (16), 109 (22), 77 (50); IR (KBr): ν = 3401, 2960, 2245, 1749, 1600, 1516, 1432, 1357, 1297, 1163 cm−1; Anal calcd for C16H17N3O3 (%): C, 64.20; H, 5.72; N, 14.04; Found (%): C, 64.03; H, 5.84; N, 13.91.
4.2.11 Methyl 3-(4-chlorophenyl)-2-cyano-3-(5-hydroxy-3-methyl-1H-pyrazol-4-yl)-propanoate (4k)
White solid, mixture of two diastereoisomers (1:1), (2.74 g, yield 86%); mp 155–156 °C; 1H NMR (300 MHz, DMSO-d6, double set of signals): δ (ppm) 1.98 (s, 3H, CH3), 2.04 (s, 3H, CH3), 3.52 (s, 3H, OCH3), 3.61 (s, 3H, OCH3), 4.35–4.42 (m, 2H, 2CH), 4.78–4.82 (m, 2H, 2CH), 7.31–7.39 (m, 6H, Ar), 7.50–7.53 (m, 2H, Ar), 10.81 (br s, 2H); 13C NMR (75 MHz, DMSO-d6, double set of signals): δ (ppm) 9.8, 9.9, 40.5, 40.7, 40.9, 41.3, 53.1, 53.2, 98.8, 99.3, 116.6, 116.7, 128.4 (4C), 129.4 (2C), 129.8 (2C), 131.7, 131.8, 137.5, 137.7, 139.5, 139.6, 159.0, 159.2, 165.7, 165.8; MS (m/z, relative intensity %): 319 ([M]+, 4), 221 (100), 185 (15), 163 (7), 128 (21), 109 (45), 75 (6), 68 (18), 59 (35), 51 (6); IR (KBr): ν = 3424, 2960, 2248, 1748, 1604, 1532, 1520, 1496, 1300, 1096 cm−1; Anal calcd for C15H14ClN3O3 (%): C, 56.35; H, 4.41; Cl, 11.09; N, 13.14; Found (%): C, 56.14; H, 4.52; Cl, 10.93; N, 12.96.
4.2.12 6-Amino-3-methyl-4-phenyl-1,4-dihydro[2,3-c]pyrazole-5-carbonitrile (5a)
White solid, (2.17 g, yield 86%); mp 244–245 °C (lit [28] mp 244–245 °C); 1H NMR (300 MHz, DMSO-d6): δ (ppm) 1.79 (s, 3H, CH3), 4.60 (s, 1H, CH), 6.83 (s, 2H, NH2), 7.16–7.34 (m, 5H, Ar), 12.09 (s, 1H, NH).
4.2.13 (4-Methoxybenzylidene)malononitrile (6)
Yellow solid, (1.80 g, yield 98%); mp 113–114 °C (lit [32] mp 113–114 °C); 1H NMR (300 MHz, CDCl3): δ (ppm) 3.92 (s, 3H, OCH3), 7.02 (d, J = 8.7 Hz, 2H, Ar), 7.65 (s, 1H, CH), 7.91 (d, J = 8.7 Hz, 2H, Ar)
Acknowledgments
The authors gratefully acknowledge the financial support of the Russian Foundation for Basic Research (Project No. 12-03-00135а).


