1 Introduction
Heterogeneous solid catalysts have gained much attention in recent years, as they possess a number of advantages, such as cleaner reaction, easier work-up, reduced reaction time, and eco-friendliness [1,2]. These considerations are currently driving our effort to develop heterogeneous organic transformations. Tungstate sulfuric acid (TSA) is an alternative to sulfuric acid that was synthesized by the reaction of anhydrous sodium tungstate with chlorosulfonic acid [3]. This new inorganic solid acid has been applied as an efficient catalyst to a variety of organic transformations [4–7].
There has been considerable interest in benzoxazole and benzothiazole derivatives, not least because of their value for a variety of industrial [8,9], biological [10,11], and medicinal chemistry uses [12,13]. Furthermore, these compounds have shown different pharmacological activities, such as anti-HIV [14,15], antitumor [16,17], anticancer [18,19], antibacterial [20,21], and anti-inflammatory [22,23] properties. The extensive applications of these compounds have prompted wide studies for their synthesis. The most frequently used synthetic method to produce benzoxazole and benzothiazole derivatives consists of the condensation of o-aminophenol or o-aminobenzenethiol with substituted aldehydes, nitriles, acyl chlorides, or carboxylic acids [13]. However, the majority of these methods suffer from certain drawbacks, for instance, the use of hazardous solvents and catalysts, high cost, long reaction times, and drastic reaction conditions.
In continuation of our interest in the use of heterogeneous solid catalysts [24–26], we wish to report herein a very simple procedure for the synthesis of benzoxazole (4) and benzothiazole (5) derivatives using TSA as a superior solid acid catalyst (Scheme 1).
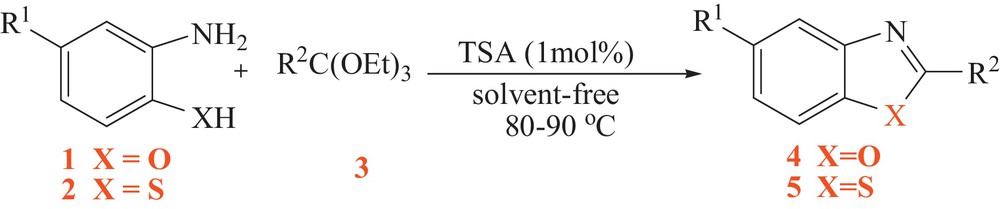
Tungstate sulfuric acid-catalyzed synthesis of benzoxazoles and benzothiazoles.
2 Results and discussion
In a first set of experiments, the solvent-free reaction of o-aminophenol (1 mmol) and triethyl orthoacetate (1.1 mmol) was performed at room temperature in the absence of the catalyst as a model reaction. No benzoxazole product was synthesized, even after 24 h. Therefore, the model reaction was investigated in the presence of different catalysts, under various conditions (Table 1).
Optimization of the reaction.
| Entry | Solvent | Catalyst (mol%) | Temperature (°C) | Time (min) | Yield (%)a |
| 1 | None | None | 90–100 | 360 | 50 |
| 2 | None | ZnCl2 (5) | 60–70 | 240 | 60 |
| 3 | None | MgBr2 (5) | 60–70 | 180 | 70 |
| 4 | None | H2SO4 (5) | 60–70 | 120 | 50 |
| 5 | None | TSA (1) | 60–70 | 25 | 87 |
| 6 | None | TSA (5) | 60–70 | 30 | 85 |
| 7 | None | TSA (10) | 60–70 | 30 | 80 |
| 8 | None | TSA (1) | 80–90 | 8 | 96 |
| 9 | None | TSA (1) | 100 | 10 | 95 |
| 10 | EtOH | TSA (1) | 70 | 120 | 65 |
| 11 | CH3CN | TSA (1) | 70–80 | 120 | 60 |
| 12 | H2O | TSA (1) | 100 | 180 | 45 |
a Isolated yields.
As it can be seen, the best results were obtained by performing the model reaction in the presence of 1 mol% of TSA at 80–90 °C under solvent-free conditions.
Then, the generality of the procedure was evaluated by the reaction of various orthoesters (3) with o-aminophenols (1) and o-aminothiophenols (2) under optimized reaction conditions. It was found that both electron-rich and electron-poor o-aminophenols (Me, Cl and NO2 as donating and withdrawing groups) reacted well in this process to afford the corresponding products in good to excellent yields. The obtained results are summarized in Table 2.
Synthesis of benzoxazole and benzothiazole derivatives in the presence of tungstate sulfuric acid (1 mol%).
| Entry | 2-Aminophenol or 2-aminothiophenol | Orthoester | Product | Time (min) | Yield (%)a | M.P. (°C) [Reference] |
| 4a | HC(OEt)3 | 7 | 92 | Oil [27] | ||
| 4b | MeC(OEt)3 | 9 | 98 | Oil [27] | ||
| 4c | EtC(OEt)3 | 4 | 98 | Oil [27] | ||
| 4d | HC(OEt)3 | 7 | 90 | 43–45 [27] | ||
| 4e | MeC(OEt)3 | 6 | 90 | Oil [27] | ||
| 4f | EtC(OEt)3 | 12 | 88 | 29–30 [27] | ||
| 4g | HC(OEt)3 | 10 | 84 | 146–148 | ||
| 4h | MeC(OEt)3 | 9 | 80 | 128–129 | ||
| 4i | EtC(OEt)3 | 10 | 93 | 150–152 | ||
| 4j | HC(OEt)3 | 4 | 96 | 36–38 [27] | ||
| 4k | MeC(OEt)3 | 7 | 97 | 53–55 [27] | ||
| 4l | EtC(OEt)3 | 10 | 90 | 58–59 [27] | ||
| 5a | HC(OEt)3 | 7 | 89 | Oil [27] | ||
| 5b | MeC(OEt)3 | 4 | 91 | Oil [27] | ||
| 5c | EtC(OEt)3 | 6 | 94 | Oil [27] |
a Isolated yields.
The products were isolated and characterized by physical and spectroscopic techniques and they were compared with authentic samples [28–32].
The probable mechanism for the formation of benzoxazoles and benzothiazoles is outlined in Scheme 2. Initially, orthoester is activated by TSA to give 6 and then 7. Next, o-aminophenol or o-aminothiophenol attacks 7, affording 8, which is then activated by the catalyst to give 9. Cyclization of 10 gives 11, which in turn is activated by the catalyst to afford the final product.
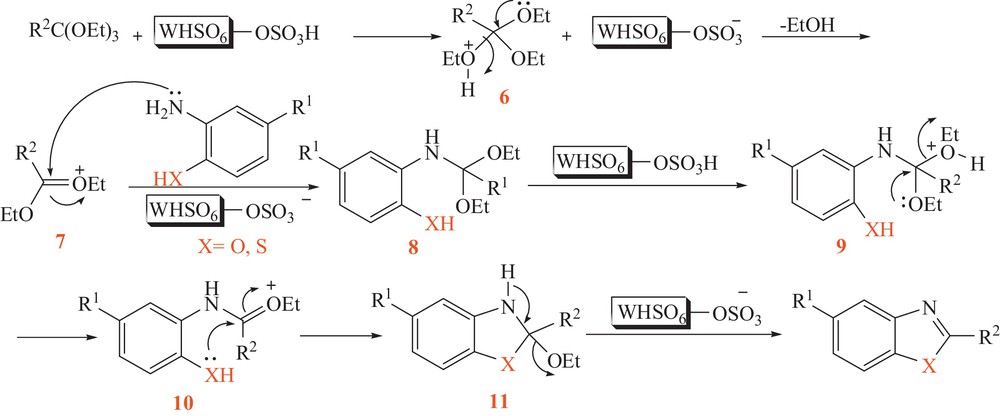
The proposed mechanism for tungstate sulfuric acid-catalyzed formation of benzoxazoles and benzothiazoles.
The reusability of the catalyst is important for the large-scale operation and from an industrial point of view. Therefore, the recovery and reusability of TSA was examined. Hence, TSA was regenerated from the model reaction by washing with chloroform and drying at 120 °C.
The major advantages of the presented protocol over the existing methods can be seen by comparing our results with those of some recently reported procedures, as shown in Table 3. For example use of o-benzenedisulfonimide required long reaction times. And use of Silica sulfuric acid as catalyst requires high temperature.
Comparison of the results for the preparation of 2-methyl benzoxazole with various used catalysts.
| Catalyst | Mol% | Time (min) | Temperature | Yield (%) [Reference] |
| o-Benzenedisulfonimide | 5 | 120–240 | R.T. | 85–93 [28] |
| Ga(OTf) | 10 | 5–90 | R.T. | 71–98 [29] |
| HBF4–SiO2 | 2 | 45–60 | R.T. | 91–97 [30] |
| Bi salts | 0.5–4 | 1–10 | 85 °C | 80–93 [31] |
| Silica sulfuric acid | 50 mg | 2–10 | 85 °C | 85–97 [32] |
3 Characterization of the catalyst
Fig. 1a shows the X-ray diffraction analysis (XRD) patterns of TSA. It was reported that high-degree mixing of W–S in chlorosulfonic acid often led to the absence of the XRD pattern for anhydrous sodium tungstate. The broad peak around 25.7° (2θ) (θ is the Bragg angle) from the smaller inset could be attributed to the linking of WO3 into the chlorosulfonic acid.

(a) Powder X-ray diffraction pattern of the tungstate sulfuric acid (TSA); (b) FT-IR spectra of H3OSO(WO2)OSO3H (TSA).
The FT-IR spectra of anhydrous sodium tungstate and TSA are shown in Fig. 2b. The spectrum of TSA shows the characteristic bonds of anhydrous sodium tungstate and chlorosulfonic acid. The wavenumbers 3406, 1820, 1725, 1702, 1620, 1290, 1060, 1005 and 860 cm−1 in the catalyst's spectra reveal bonds in both anhydrous sodium tungstate and the –OSO3H group. Hence, the titration of the catalyst with NaOH (0.1 N) was done. Firstly, 1 mmol of catalyst was dissolved in 100 mL of water. Therefore, the titration with NaOH (0.1 N) in the presence of phenolphthalein was carried out. At the endpoint of the titration, 4 mmol of the titrant was consumed. Also, based on potentiometric data, the pKa1 value for TSA is 3.17 and pKa2 is 8.78. The result of the catalyst's titration showed four acidic valences because of hydrolysis in aqueous solvent. The X-ray fluorescence (XRF) data of TSA indicates the presence of WO4 and SO3 in this catalyst (Table 4).

Reusability study of the catalyst in the model reaction.
XRF data of HO3SO(WO2)OSO3H (TSA).
| Compound | Concentration (%W/W) |
| WO4 | 19.49 |
| SO3 | 0.317 |
| Na2O | 0.190 |
| Cl | 0.056 |
| CuO | 0.023 |
| Fe2O3 | 0.015 |
| CaO | 0.014 |
| LOIa | 79.82 |
| Total | 99.93 |
a Loss on ignition.
4 Conclusion
In conclusion, TSA was found to be a mild and efficient catalyst for the formation of benzoxazoles and benzothiazoles. The use of this reusable catalyst under solvent-free conditions has made this protocol practical, environmentally friendly and economically attractive. The simple work-up procedure, the mild reaction conditions, the short reaction times, the high yields of products and the non-toxicity of the catalyst are other advantages of the present method.
5 Experimental
5.1 General
XRD patterns were obtained with a Philips X Pert Pro X diffractometer operated with a Ni-filtered Cu Kα radiation source. XRF spectroscopy spectra were recorded with the X-ray fluorescence analyzer S4 Pioneer, Bruker, Germany. The chemicals were purchased from Aldrich, Fluka and Merck chemical companies and freshly used without purification. The products were isolated and identified by their spectral data. IR spectra were recorded on a FT-IR JASCO-680 using KBr disks. The 1H NMR and 13C NMR spectra were recorded with a Bruker 400 Ultrashield® (400 MHz), with CDCl3 as the solvent.
5.2 Preparation of the catalyst
TSA was prepared via the previously reported procedure [3]. First, anhydrous sodium tungstate (2 mmol, 5.876 g) was added to dry n-hexane (25 mL) in a 100-mL round bottom flask, equipped with an ice bath and overhead stirrer. Chlorosulfonic acid (4 mmol, 0.266 mL) was then added dropwise to the flask during 30 min and stirred for 1.5 h. Afterwards, the reaction mixture was gradually poured into 25 mL of chilled distilled water under agitation. The TSA was separated as a yellowish solid by filtration, washed with distilled water five times till the filtrate showed a negative test for the chloride ion, and dried at 120 °C for 5 h. The yield of the obtained yellowish catalyst proved to be 98% after it was decomposed at 285 °C.
5.3 Reusability of the catalyst
At the end of the reaction of o-aminophenol (1 mmol), triethyl orthoacetate (1.1 mmol) and TSA (1 mol%), the catalyst was filtered, washed with chloroform, and dried at 120 °C for 1 h. Using the recycled catalyst for five consecutive times in the model reaction afforded the product with a gradual decrease in the reaction yield. The results of these observations are shown in Fig. 2.
5.4 General procedure for the synthesis of benzoxazole and benzothiazole derivatives
To a mixture of orthoester (1.1 mmol) and o-aminophenol or o-aminothiophenol (1 mmol) was added TSA (1 mol%). The mixture was stirred at 80–90 °C for the appropriate time according to Table 4. After the completion of the reaction (as indicated by TLC), the mixture was diluted with chloroform (10 mL) and the catalyst was separated by filtration. Further purification was achieved by column chromatography.
5.5 Selected spectral data
5.5.1 2-Methylbenzoxazole (4b)
Colorless oil, FT-IR: νmax (KBr) = 3050, 2900, 1617, 1575, 1450, 1160 cm−1, 1H NMR (400 MHz, CDCl3): δ = 2.61 (s, 3H, CH3), 7.27 (m, 2H, CH arom), 7.44 (m, 1H, CH arom), 7.63 (m, 1H, CH arom) ppm, 13C NMR (100 MHz, CDCl3): δ = 14.2, 110.7, 119.5, 124.0, 124.4, 141.5, 150.8, 163.5 ppm.
5.5.2 2,5-Dimethylbenzoxazole (4e)
Colorless oil, FT-IR: νmax (KBr) = 3010, 2950, 1575, 1450, 1260, 610 cm−1, 1H NMR (400 MHz, CDCl3): δ = 2.39 (s, 3H, CH3), 2.55 (s, 3H, CH3), 7.02 (d, J = 8.4 Hz, 1H, CH arom), 7.27 (d, J = 8.1 Hz, 1H, CH arom), 7.39 (s, 1H, CH arom) ppm, 13C NMR (100 MHz, CDCl3): δ = 14.1, 22.5, 109.6, 119.5, 125.3, 133.4, 141.6, 149.1, 163.4 ppm.
5.5.3 5-Nitrobenzoxazole (4g)
Yellow solid, mp 146–148 °C, FT-IR: νmax (KBr) = 3055, 1620, 1560, 1462, 1169 cm−1, 1H NMR (400 MHz, CDCl3): δ = 7.27 (m, 1H, CH arom), 8.08 (m, 1H, CH arom), 8.18 (m, 1H, CH arom), 8.53 (s, 1H) ppm, 13C NMR (100 MHz, CDCl3): δ = 111.42, 117.19, 121.75, 140.49, 145.47, 153.44, 155.23 ppm.
5.5.4 2-Methyl-5-nitrobenzoxazole (4h)
White solid, mp 128–129 °C, FT-IR: νmax (KBr) = 3100, 3000, 1610, 1572, 1515, 1445, 1340, 1160 cm−1, 1H NMR (400 MHz, CDCl3): δ = 2.52 (s, 3H, CH3), 7.39 (d, J = 9.2 Hz, 1H, CH arom), 8.09 (dd, 3JHH = 8.8 Hz, 4JHH = 2 Hz, 1H, CH arom), 8.36 (d, J = 2 Hz, 1H, CH arom) ppm, 13C NMR (100 MHz, CDCl3): δ = 14.9, 110.4, 115.8, 120.7, 141.9, 145.1, 154.5, 167.1 ppm.
5.5.5 5-Chlorobenzoxazole (4j)
White solid, mp 36–38 °C, FT-IR: νmax (KBr) = 3100,1600, 1576, 1445, 1160 cm−1, 1H NMR (400 MHz, CDCl3): δ = 7.18 (d, J = 8.4 Hz, 1H, CH arom), 7.32 (d, J = 8.8 Hz, 1H, CH arom), 7.59 (s, 1H, CH arom), 7.92 (s, 1H) ppm, 13C NMR (100 MHz, CDCl3): δ = 111.7, 120.6, 126.2, 130.2, 141.4, 148.6, 153.7 ppm.
5.5.6 5-Chloro-2-ethylbenzoxazole (4l)
White solid, mp 59–61 °C, FT-IR: νmax (KBr) = 3050, 2800, 1600, 1560, 1440, 1160 cm−1, 1H NMR (400 MHz, CDCl3): δ = 1.26 (t, J = 7.6 Hz, 3H, CH3), 2.76 (q, J = 7.6 Hz, 2H, CH2), 7.07 (m, 1H, CH arom), 7.2 (d, J = 8.4 Hz, 1H, CH arom), 7.45 (d, J = 1.2 Hz, 1H, CH arom) ppm, 13C NMR (100 MHz, CDCl3): δ = 10.7, 22.1, 110.9, 124.6, 124.9, 129.4, 142.5, 149.4 ppm.
5.5.7 2-Methylbenzothiazole (5b)
Pale yellow oil, FT-IR: νmax (KBr) = 3050, 2880,1617, 1575, 1450, 1160 cm−1, 1H NMR (400 MHz, CDCl3): δ = 2.84 (s, 3H, CH3), 7.34 (t, J = 7.6 Hz, 1H, CH arom), 7.44 (t, J = 8.1 Hz, 1H, CH arom), 7.81 (d, J = 8.0 Hz, 1H, CH arom), 7,95 (d, J = 8.0 Hz, 1H, CH arom) ppm, 13C NMR (100 MHz, CDCl3): δ = 20.1, 121.3, 122.6,124.3, 126.1, 135.6, 153.3, 166.8 ppm.
Acknowledgement
We acknowledge the research council of Yasouj University.


