1 Introduction
Local pain management is the most critical point of patient care in dentistry and medicine [1] and anesthetics and anti-inflammatory drugs are commonly used [2]. These drugs develop therapeutic effects by their anti-inflammatory, analgesic, and antipyretic activities [3].
The systemic drug administration presents disadvantages due to the spreading of drugs throughout the body, which causes unwanted toxic effects to healthy organs [4]. Local controlled drug delivery received increasing attention since it presents a solution to characteristic problems of systemic drug administration [5–7].
Collagen sponges are safe and well-characterized supports for drug delivery systems, [8] since they are biocompatible and biodegradable biomaterials. The most used drugs in local topical administration are: antibiotics, steroids, cholinergic, anticoagulants, immuno-depressants [5,9]. Drug delivery systems in form of collagen sponges with doxycycline [10–12], gentamicin [13], triphala [14], tobramycin or ciprofloxacin [15] were studied successfully in vitro or in vivo.
Niflumic acid (NA) is an analgesic and anti-inflammatory agent, which could be used in pain management in dentistry and medicine. In our previous study, we developed some modern wound dressings such as controlled drug delivery systems based on collagen. For these materials, the maximum concentration of niflumic acid that did not produce toxicity was established [16]. Based on these results, the aim of this study was to continue our research in order to improve the solubility of the drug in aqueous solutions, the stability of the drug delivery systems and to control the release of niflumic acid. In this paper, a series of five spongious (3D) drug delivery systems based on collagen and niflumic acid cross-linked with different concentrations of glutaraldehyde were obtained and characterized by FTIR spectroscopy, water absorption and collagenase degradation. The niflumic acid release from cross-linked collagen spongious forms was also investigated and the kinetic mechanism was discussed.
2 Materials and methods
2.1 Materials
Type-I collagen of bovine origin was provided by Collagen Department of Division Leather and Footwear Research Institute; it had been extracted by the currently used technology previously described [9]. The collagen (Coll) was obtained as a gel in its native form with fibrillar structure with an initial concentration of 2.5%, pH 2.5 and free of fat and ash. Niflumic acid (NA) was purchased from ICN Biomedicals Inc. (USA) and glutaraldehyde (GA) from Merck (Germany). The sodium hydroxide and the phosphate buffer solutions (PBS), pH = 7.4 were of analytical grade. All the chemicals used in this work were of analytical grade; water was distilled.
2.2 Preparation of niflumic acid - collagen delivery systems
The pH of collagen gel was adjusted at 7.4 under mechanical stirring with a 1 M sodium hydroxide solution. Niflumic acid 1 g/L was embedded into the collagen gel and then both collagen reference and collagen with niflumic acid were cross-linked with different concentrations of glutaraldehyde (GA) (0.25, 0.5, 0.75 and 1% with respect to the dry substance). All samples have a concentration of 4% of niflumic acid with respect to the dry substance (0.1% with respect to the collagen gel). The gels were then freeze-dried using the freeze-dryer Delta 2-24 LSC (Martin Christ, Germany), as previously described [17], in order to obtain 3D collagen systems (spongious forms).
2.3 FTIR analysis
Infrared analysis was performed using a PerkinElmer Spectrum 100 FTIR spectrophotometer in the attenuated total reflection mode on the spongious materials in order to evidence the presence of bonds between anti-inflammatory drug and collagen as well as the structural changes.
2.4 Water absorption analysis
Water absorption was evaluated as previously described [16]. Briefly, spongious materials with and without NA were weighted as such, and after immersion in water, at different time intervals until a stable mass was obtained. Then the percentage of water absorbed in spongious materials was evaluated.
2.5 In vitro kinetic studies
The in vitro niflumic acid release from collagen sponges was determined using a paddle dissolution equipment, as previously described [18,19]. Briefly, inside the apparatus release vessel, a transdermal sandwich device with the sponge sample is fitted. The phosphate buffer solution, pH 7.4, maintained at 37 °C, was used as release medium.
During the experiments (approximately 4 h), aliquots of 5 mL were withdrawn at predetermined time periods from the release medium and replaced with an equal volume of fresh phosphate buffer solution. The absorbance at 287 nm (PerkinElmer Spectrophotometer) was recorded for each sample and the released niflumic acid amount was evaluated based on the calibration curve previously determined () [16].
3 Results and discussion
3.1 FTIR spectra
Figs. 1–3 and Table 1 present the IR spectra and the main wave numbers obtained for samples of collagen, niflumic acid and collagen with niflumic acid and cross-linking agent.
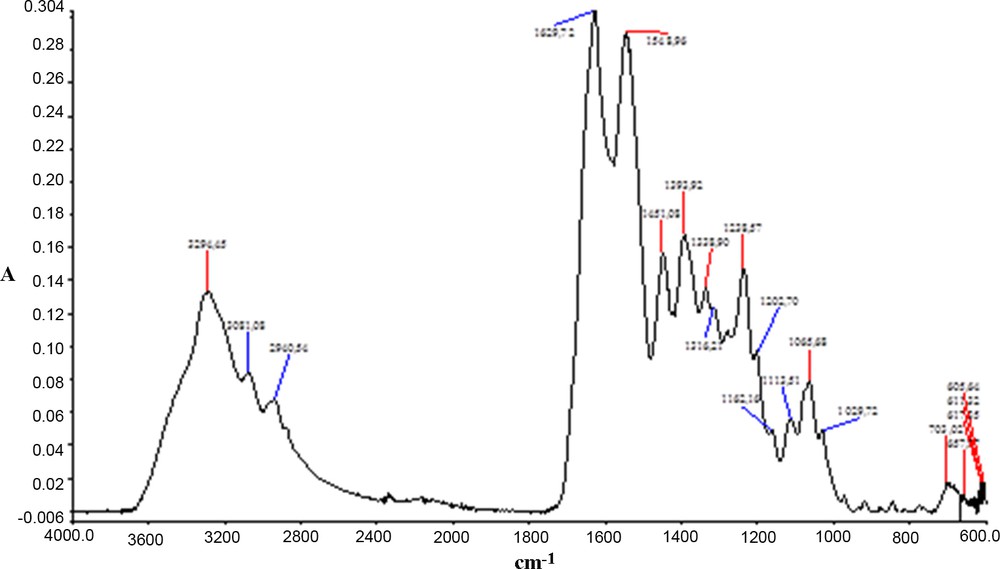
Infrared spectrum of a collagen scaffold. Color available online.

Infrared spectrum of niflumic acid. Color available online.

Infrared spectrum of a scaffold with collagen, cross-linking agent and niflumic acid. Color available online.
Wave numbers and band assessments for IR spectra of raw and synthetised materials.
| Wave number v [cm−1] | Band assessments | Wave number v [cm−1] | Band assessments | |||||
| Scaffold | Powder | |||||||
| CG | CG NA | CG NA GA 0.25% | CG NA GA 0.5% | CG NA GA 0.75% | CG NA GA 1% | NA | ||
| 3294 | 3295 | 3294 | 3300 | 3292 | 3295 | |||
| 3081 | 3080 | 3080 | 3080 | 3085 | 3085 | δN–H | 3012 | νO–H |
| 2951 | 2961 | 2961 | 2961 | 2956 | 2972 | 2860 | νCH | |
| 1630 | 1631 | 1632 | 1634 | 1631 | 1627 | νCO (amide I) | 1662 | νCO |
| 1607 | C6H6 | |||||||
| 1589 | 1592 | 1587 | 1591 | 1592 | δN-H | 1581 | δN-H | |
| 1549 | 1559 | 1559 | 1559 | 1558 | 1559 | δN–H; νC–N (amide II) | 1531 | νCC aromatic |
| 1516 | 1520 | 1516 | 1516 | 1516 | νCC aromatic | |||
| 1451 | 1447 | 1447 | 1447 | 1447 | 1447 | COO− | 1423 | δCH |
| 1398 | 1387 | 1387 | 1387 | 1387 | 1390 | N–H | 1394 | N–H |
| 1339 | 1332 | 1332 | 1331 | 1331 | 1331 | C–C; νC–N (amide III) | 1326 | δO–H acid |
| 1237 | 1235 | 1235 | 1235 | 1235 | 1234 | νC–N | 1175 | νC–F |
| 1117 | 1118 | 1118 | 1118 | νC–OH acid | 1146 | νC–F | ||
| 1081 | 1068 | 1068 | 1068 | 1068 | 1068 | νC–OH | 1104 | νC–OH acid |
| 1031 | 1031 | 1031 | 1031 | 1031 | 1034 | νCO | 1066 | νC–OH acid |
| 975 | 972 | 972 | 972 | 975 | δC–H aromatic in plane | 973 | δC–H aromatic in plane | |
| 779 | 778 | 777 | 778 | 777 | δC–H aromatic trisubstituted 1, 2, 3 out of plane | 770 | δC–H aromatic trisubstituted 1, 2, 3 out of plane | |
| 697 | 697 | 697 | 697 | 697 | δC–H aromatic disubstituted 1,3 out of plane | 696 | δC–H aromatic disubstituted 1,3 out of plane |
In the polymeric structure of collagen (Fig. 1) can be easily recognized the bands for amide I, amide II, and amide III at 1630, 1549, and 1339 cm−1 respectively, accompanied by specific bands for associated amides at 3294 and 3081 cm−1.
Niflumic acid (Fig. 2) has a large band around 3000 cm−1 specific to νO–H, which is accompanied by an intensive νCO vibration at 1662 cm−1. Other numerous intensive bands appear between 1140 and 1170 cm−1; they are characteristic of the νC–F vibration. Intensive bands within the low frequency range (760–980 cm−1), specific to aromatic compounds, are also evidenced.
Glutaraldehyde—the cross-linking agent—, being in a rather low amount (< 1%) and without specific bands, cannot be distinguished from the collagen polymeric matrix. However, the presence of the active compound, niflumic acid, can be without a doubt assessed by means of its “fingerprints” at 1592 cm−1 (δN–H), 1521 cm−1 (νCC aromatic), and of the bands amplified at 1332 cm−1 (δO–H acid), 1118 and 1068 cm−1 (νC–OH acid).
3.2 Water absorption
Fig. 4 shows the results obtained for water absorption on different collagen-based scaffolds. One can see that the presence of niflumic acid as well as of the cross-linking agent has importance on water absorption. Water absorption is generally increasing with decreasing the content in cross-linking agent. In all the cases, the presence of the anti-inflammatory drug favors water absorption. Taking into account the final application of these materials, water absorption is not a desired phenomenon; however further drug release measurements demonstrate that the amount of cross-linking agent is important.
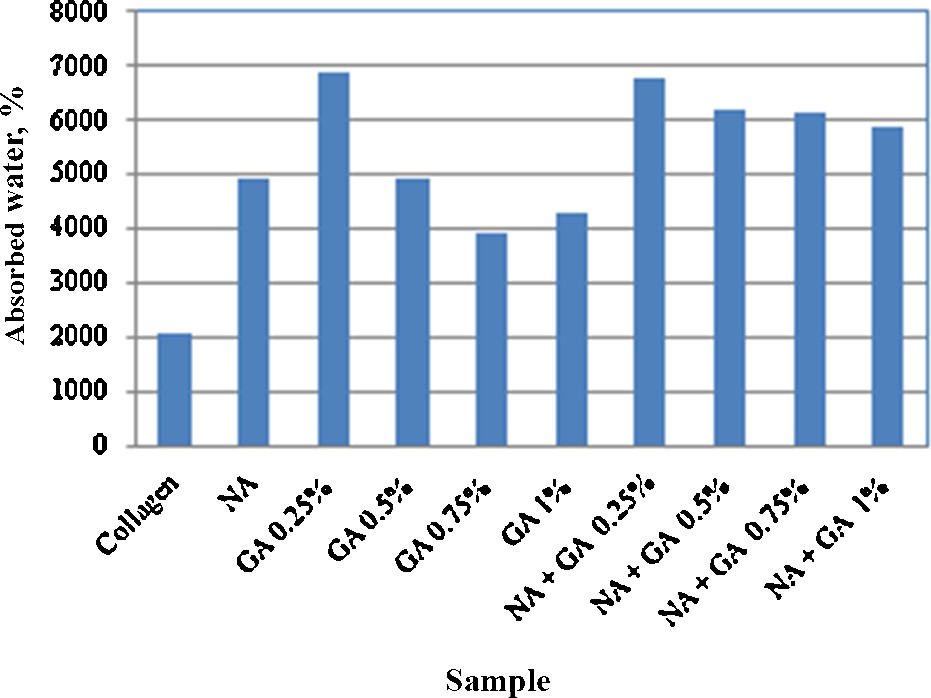
Water absorption of different collagen based scaffolds (NA: niflumic acid; GA: cross-linking agent).
3.3 Drug release kinetic study
The cumulative kinetic profiles corresponding to the released drug percent as a function of time recorded for the collagen sponges, free and cross-linked with different amounts of GA, are illustrated in Fig. 5, highlighting the influence of the cross-linking agent concentration on the released NA percent at various periods of time.

Niflumic acid release as function of cross-linking agent concentration. Color available online.
It can be seen that the shape of the release patterns is quite similar and that it does not depend on the quantity of cross-linking agent. This means that niflumic acid is released in a similar manner for any glutaraldehyde concentration, but the percent of niflumic acid released is inversely proportional to the cross-linking agent's amount. This behavior is due to the strong chemical cross-linking determined by the GA increased amount, which implies a slower NA release from the tested matrices. The highest released drug percent was obtained for the sample without cross-linking agent (96.05%), while the smallest one was obtained for samples with the highest glutaraldehyde content (65.34%), recording a decrease by 1.47 times. The presence in the formulation of the minimum GA amount (0.25%) determines a decrease of the released NA percent of about 6.53% compared to the corresponding un-cross-linked matrix. In comparison with the matrices having 0.50 and 0.75% GA, the released drug percent for the corresponding un-cross-linked matrix is 1.15 and, respectively, 1.20 times higher.
In order to analyse the niflumic acid release mechanism from the designed collagen sponges, the following power law model (Eq. (1)) was used:
| (1) |
where mt/m∞ is the fraction of drug released at time t, k is the kinetic constant and n is the release exponent that can be related to the drug transport mechanism [19]. The kinetic parameters n and k are listed in Table 2.
Values for kinetic parameters characteristic of the power law model; niflumic acid percent released and Pearson coefficient, R.
| Niflumic acid collagen sponge | Kinetic constant k, (1/minn) | Release exponent, n | Percent released (%) | R |
| NA + GA 0.00% | 0.333 | 0.201 | 96.05 | 0.9941 |
| NA + GA 0.25% | 0.270 | 0.228 | 89.78 | 0.9925 |
| NA + GA 0.50% | 0.229 | 0.251 | 83.55 | 0.9837 |
| NA + GA 0.75% | 0.154 | 0.309 | 79.65 | 0.9921 |
| NA + GA 1.00% | 0.139 | 0.294 | 65.34 | 0.9894 |
As observed in Table 2, the values of n were smaller than 0.5, suggesting a non-Fickian diffusion behavior. The release profiles were better fitted with a power-law model than with Higuchi's model, which is true for n = 0.5, the last model corresponding to a Fickian diffusion transport mechanism. The values recorded for Pearson coefficient (R) for the above kinetic models are also listed in Table 2 and confirm the non-Fickian diffusion behavior.
Drug formulations for dental applications should allow the active substance to be released over a long period of time. For our formulations this period is increasing with glutaraldehide concentration increasing, being 2 h for scaffolds without cross-linking agent, 5 h for sponges with 0.25% glutaraldehide, 6 h (resp. 7 h) for materials with 0.5% (resp. 0.75%) glutaraldehide, and 13 h for materials with 1% cross-linking agent. On the other hand, one major advantage of these formations is the collagen itself, which was proved to be a safe matrix for an application to dental materials.
Corroborating the results obtained for water absorption and drug release experiments and taking into account the fact that a compromise solution should be found in designing a drug: moderate rate of release with a high quantity of drug and an acceptable water absorption, suggest that the optimum concentration for the cross-linking agent is 0.5%.
4 Conclusions
The aim of this study was to obtain materials based on collagen for biomedical applications, especially in dentistry. In this respect, an anti-inflammatory drug was embedded into collagen sponges. The stability of these materials was assured by glutaraldehyde as a cross-linking agent.
Measurements related to hydrolysis and enzymatic degradation confirm the previous studies [16] showing that glutaraldehyde inhibits collagen degradation, while niflumic acid addition increases this phenomenon in case of enzymatic degradation (with collagenase); water absorption is increasing when the quantity of cross-linking agent decreases, whereas the presence of niflumic acid enhances water absorption.
The kinetic profiles determined for niflumic acid release from the collagen matrices formulated with various glutaraldehyde amounts highlight the influence of the cross-linking agent on both release constant and released drug percent. A non-Fickian kinetic mechanism was also revealed for drug release from the designed sponges.
The released niflumic acid percent can be controlled by modulation of the amount of cross-linking agent, thus allowing the monitoring of the delivered drug optimum amount in relation with the application site and therapeutic recommendations.


