1 Introduction
The development of fluorescent sensors for the detection of heavy metal ions in aqueous solutions that are harmful for the environment is of increasing interest [1–4]. In this rapidly developing field, fluorescent detection has become one of the most exploited techniques owing to its high sensitivity and ease of operation [5,6]. It is worth to mention that the word “sensor” refers to a device that is capable of responding to the presence of an analyte such as a metal ion in a reversible and continuous manner. Sensors producing an irreversible response to the presence of heavy metal ions are called probes. The detection of heavy metal ions is mainly based on the use of a chromophore capable of binding with the ions upon which a fast response by a change in the absorbance or fluorescence of such chelators takes place. This means that the chromophore acts as a transducer for the chemical species that cannot be determined directly by optical means [7–9]. Thus, sensors based on ion-induced changes in fluorescence appear to be particularly attractive due to their simplicity, high sensitivity, high selectivity, and instantaneous response [10].
The immobilization of a fluorescent chromophore (fluorophore) onto a solid support such as a polymer matrix has advantages for practical applications due to easy recovery and reusability. Various polymeric membranes have been used as supporting matrices for the preparation of optical chemical sensors in the literature. In principle, the immobilization of a fluorophore either by chemical or physical bonds with a plasticized polymer facilitates sensor–analyte interactions and produces a distinctive change in the optical signal. The selection of the polymer matrix for the ion-sensitive thin film is governed by parameters such as permeability of the analyte, mechanical stability, and its suitability for chromophore immobilization [11]. Basic principles, techniques, and recent studies of optical ion sensing have been described [12,13] and reviewed [14–17].
Cellulose triacetate (CTA) is a transparent semi-synthetic biodegradable biopolymer, widely used in different applications such as hemodialysis, reverse osmosis membranes, and liquid crystal displays [18]. Previous studies have shown that CTA is an effective polymer matrix for the transport of some transition metal ions after being blended with a plasticizer and an ionophore [19–21]. It has been reported that the use of PEG 600 as an additive for CTA membranes promoted a higher resistance to pressure of the prepared membranes and increased pure water flux through the membranes, as well as the ion diffusion coefficient [22]. Therefore, it was hypothesized that mixing PEG 600 as the plasticizer with CTA would help better ion–sensor interaction by increasing the polarity of the polymer matrix.
8-Hydroxyquinoline (8-HQ) was selected in this study owing to its on–off fluorescence response upon metal ion binding [23]. Sensors based on 8-HQ moiety have recently been reported [24–26]. Recent studies indicated that the on–off fluorescence of 8-HQ upon its interaction with metal ions has been attributed to a proton transfer mechanism in which 8-HQ is weakly or nonfluorescent (off) due to an excited-state proton transfer (ESPT); upon binding with the metal ion, fluorescence appears (on), due to the inhibition of the ESPT process [27]. To the best of our knowledge, a chromophoric thin film based on CTA-immobilized 8-HQ has not been reported. In this work, chromophoric thin films composed of CTA as the polymer matrix, PEG 600 as the plasticizer and 8-HQ as the chromophore are investigated. These films were characterized by thermal analysis (TGA), FTIR, UV-visible and fluorescence spectra before and after metal ion binding.
2 Experimental
2.1 General
Cellulose triacetate (CTA), polyethylene glycol 600 (PEG 600), 8-hydroxyquinoline (8-HQ), dichloromethane (DCM), AlCl3·H2O, ZnCl2, and TlCl (thallium (I) chloride) were of the highest purity available, purchased from BDH Chemicals Ltd Poole, England, and used as received.
2.2 Thin film preparation
Six thin films, referred to as CTA, CTA-PEG1, CTA-PEG2, CTA-8-HQ, CTA-PEG1-8-HQ, and CTA-PEG2-8-HQ with the composition shown in Table 1, have been prepared by the solution casting method. The first set of films (CTA, CTA-PEG1, and CTA-PEG2) and the second set of films (CTA-8-HQ, CTA-PEG1-8-HQ, and CTA-PEG2-8-HQ) were prepared at different PEG 600 contents and in the absence and presence of 8-HQ, respectively as follows. The film solutions for the first set were prepared by dissolving 1000 mg of CTA and different PEG 600 (0, 500, and 1000 mg) in 32 ml of DCM for making films CTA, CTA-PEG1 and CTA-PEG2, respectively. Similarly, the film solutions for the second set were prepared by dissolving 1000 mg of CTA, different PEG 600 (0, 500, and 1000 mg) and different 8-HQ (5.8, 8.7, and 11.6 mg) in 32 mL of DCM for making films CTA-8-HQ, CTA-PEG1-8-HQ, and CTA-PEG2-8-HQ, respectively. The different amounts used from 8-HQ produced the same fluorophore concentration (0.58% w/w based on the weight of polymer matrix). All solutions were homogenized at room temperature after having been stirred for one hour. The films were made by casting ca. 20 g of the homogenized mixture in a Petri dish (80 × 10 mm). The solution was dried at 50 °C overnight and the films obtained were of thickness ca. 0.15 mm. The films were cut into strips of dimension 9 × 30 mm so as to be used for UV-visible and fluorescence measurements.
Sample description.
| Film | Composition |
| CTA | 1000 mg CTA/32 mL DCM |
| CTA-PEG1 | 1000 mg CTA + 500 mg PEG/32 mL DCM |
| CTA-PEG2 | 1000 mg CTA + 1000 mg PEG/32 mL DCM |
| CTA-8-HQ | The composition of all films are the same as above but in the presence of 8-HQ (0.58% w/w of polymer matrix) |
| CTA-PEG1-8-HQ | |
| CTA-PEG2-8-HQ |
2.3 Absorption and fluorescence spectra
Absorption and fluorescence emission spectra of films were recorded in quartz cells. The films were placed in diagonal position in the quartz cell. For the fluorescence measurements, the quartz cell was filled with the salt solution (5 × 10−5 M) and the strip was immersed, then the fluorescence was measured after 5 min as a constant response time. The excitation wave length was 350 nm and the slit width was set at 5.0 nm for both excitation and emission. Absorption spectra were measured on a UV-1650 PC Shimadzu spectrophotometer. Fluorescence spectra were measured on a RF-5301 PC Shimadzu spectrofluorophotometer and are uncorrected.
2.4 ATR–FTIR
The Attenuated Total Reflectance-Fourier Transform Infrared (ATR–FTIR) spectra of thin films were measured in the 4000–650 cm−1 regions using a PerkinElmer spectrum 100 FTIR spectrometer.
2.5 Thermal analysis
Thermogravimetric analysis (TGA) was carried out on a Shimadzu TGA-50H thermogravimeter analyzer and the samples were heated from room temperature to 650 °C at a rate of 20 °C/min under an inert nitrogen atmosphere.
3 Results and discussion
3.1 ATR–FTIR
Infrared spectra showed the following characteristics bands of cellulose triacetate (CTA) in Fig. 1: –OH stretch at 3647 cm−1 free OH, aliphatic C–H stretch at 2920 and 2850 cm−1, C = O stretch carbonyl (ester) at 1740 cm−1, C–O–C stretch peaks at 1035, 1120 and 1220 cm−1, and C–H bending at 1372 and 1435 cm−1 for CH3 and CH2, respectively. Upon mixing cellulose triacetate with PEG 600, special changes were observed as indicated in Fig. 1, in which the O–H stretch has become broad owing to the hydrogen bond imposed by PEG macromolecules. In addition, C–O–C stretch peaks together with the aliphatic C–H stretch have become more intense in the presence of PEG. However, the presence of 8-HQ in the film CTA and CTA-PEG2 is not easily seen in both films, as the functional groups of 8-HQ are expected to be overlapped with those of CTA and/or CTA-PEG2.

ATR–FTIR of different thin films in the absence and in the presence of 8-HQ (color online).
3.2 Thermal analysis
The TGA thermographs of CTA-based films as a function of different PEG contents are shown in Fig. 2. As shown in Fig. 2, all films reveal a similar pattern, with two stages of weight loss. The first stage is due to loss of water and other volatile organic matters, while the second is due to decomposition of CTA [22,28]. The relative thermal stabilities of the different films can be assessed by comparing the weight losses in a 50–650 °C temperature range. It is evidenced that 5 wt% of the total weight of CTA-PEG1 film is decomposed for temperatures higher than 300 °C, whereas CTA-PEG2 film starts to decompose at 326 °C. Compared with CTA, however, the decomposition of 5 wt% of the total weight takes place at 346 °C. This result indicates that the presence of PEG 600 has induced structural changes in the films.

TGA of CTA, CTA-PEG1 and CTA-PEG2 thin films (color online).
3.3 Absorption and fluorescence measurements
The absorption spectra of different films containing 0.58% (w/w) of 8-HQ is shown in Fig. 3. Even though the concentration of 8-HQ is the same in the three films, their absorption values at the maximum wavelength (309 nm) are dependent on the film composition: they show increasing absorbances in the order 0.46 (CTA-8-HQ) < 0.62 (CTA-PGE1-8-HQ) < 0.98 (CTA-PEG2-8-HQ). This result may be attributed to the effect of PEG content on the optical properties of the film. It has been reported that PEG 600 is an effective plasticizer as well as a pore-forming agent if blended with a polymer such as cellulose acetate [29]. Thus, it would be rational to expect more hydrophilic environment and more transparency of the film as the PEG content increases, and as a consequence the optical absorption of the films will also increase.

Absorption spectra of 8-HQ in different polymer films (color online).
The fluorescence spectra of different films containing 0.58% (w/w) of 8-HQ are shown in Fig. 4. It is interesting to see a clear fluorescence of 8-HQ-containing CTA, whereas CTA-PEG2-8-HQ has a very weak and non-structured fluorescence. Previous studies indicated that the fluorescence spectra of 8-HQ present a non-structured fluorescence band in the 360–460 nm region, whose intensity and wavelength are strongly affected by the nature of the solvent [30]. It was concluded that the weak fluorescence of 8-HQ is due to the ESPT process, which get enhanced in protic solvents owing to the presence of intermolecular hydrogen bonds between the solvent molecules and 8-HQ [30,31]. Therefore, it is anticipated that the presence of PEG in the composite (CTA-PEG2-8-HQ) gives it a protic character different from that in its absence (CTA-8-HQ), and thus, it is expected that the intermolecular hydrogen bonds in the former film are more numerous than in the latter. These enhanced hydrogen bonds due to the presence of PEG in the matrix play a major role in quenching the fluorescence of 8-HQ by the mechanism of radiationless relaxation via the ESPT process. Scheme 1 depicts the inhibition of ESPT upon chelation of 8-HQ with metal ions using CTA-PEG film. It is worth noting that the fluorescence of CTA-PEG2-8-HQ is similar to the fluorescence of CTA-PEG1-8-HQ.
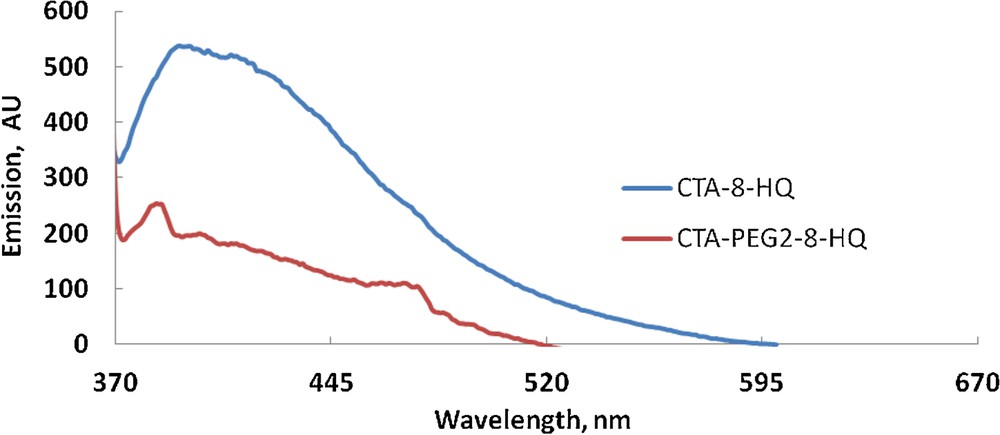
Emission spectra of 8-HQ in different polymer films (color online).
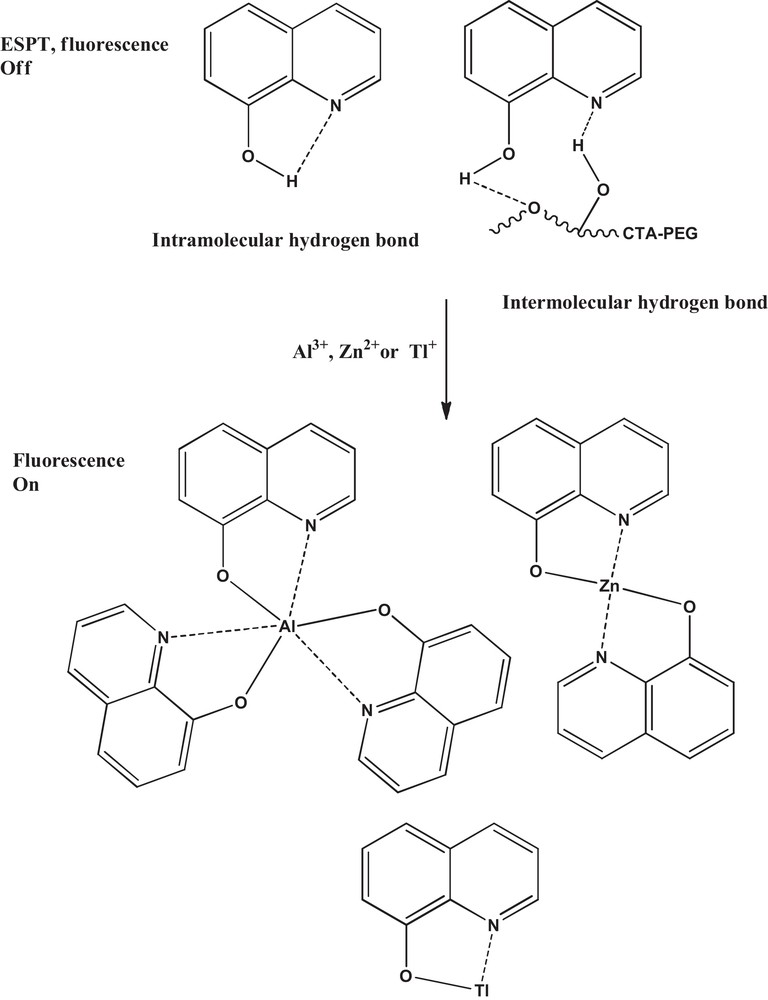
Inhibition of ESPT upon chelation of 8-HQ with metal ions using CTA-PEG film (color online).
Fluorescence detection spectra of metal ions (Al3+, Zn2+, Tl+, 5 × 10−5M) are shown in Figs. 5–7. Fig. 5 shows a comparative fluorescence detection of Al3+ using CTA-8-HQ, CTA-PEG1-8-HQ and CTA-PEG2-8-HQ. As can be seen, CTA-8-HQ and CTA-PEG1-8-HQ reveal only the fluorescence of 8-HQ, whereas, CTA-PEG2-8-HQ shows both fluorescence, the free 8-HQ at ca. 400 nm and fluorescence of the chelated one at ca. 520 nm, indicating the suitability of this film for Al3+ detection.
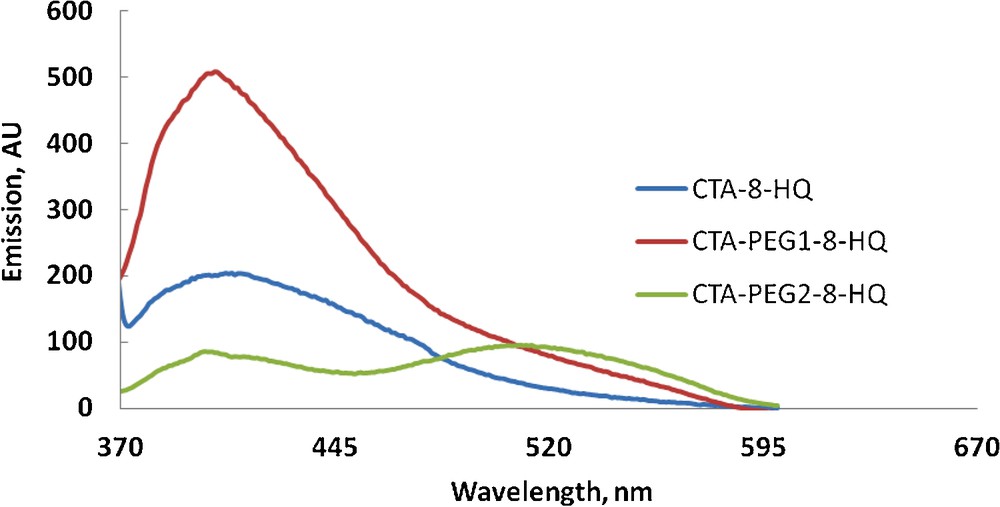
Fluorescence detection of 5 × 10−5 M of AlCl3with different polymeric films (color online).
Fluorescence detection of 5 × 10−5 M of ZnCl2 with different polymeric films (color online).
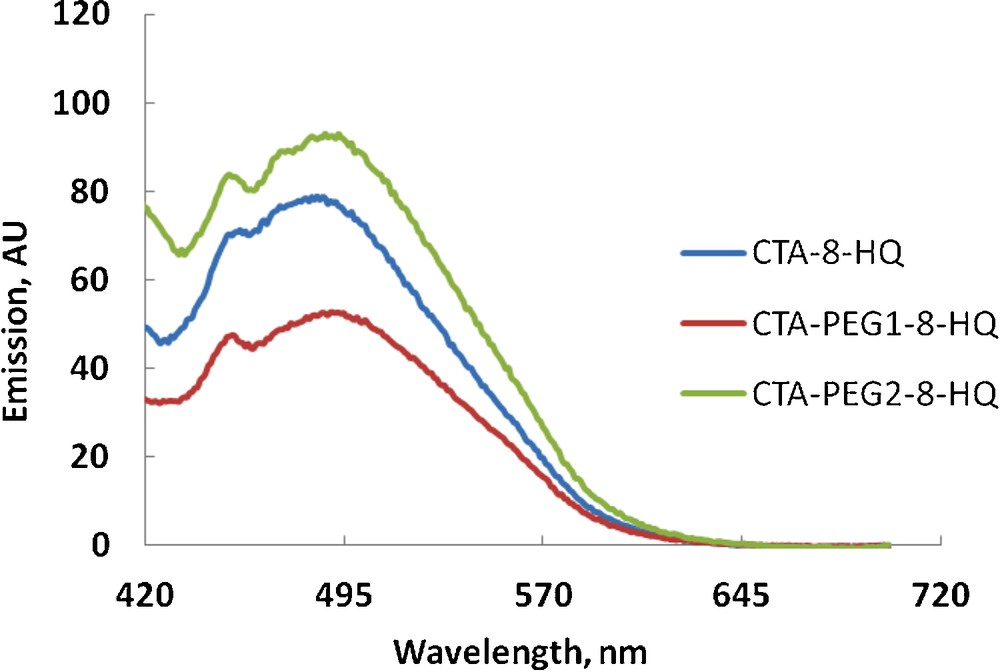
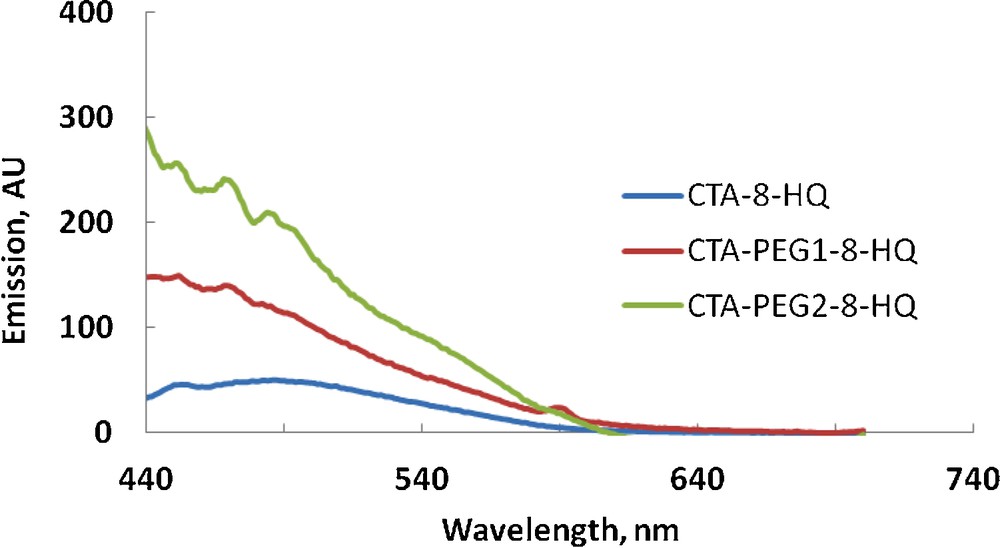
Fluorescence detection of 5 × 10−5 M of TlCl with different polymeric films (color online).
Fig. 6 shows a comparative fluorescence detection of Zn2+ using CTA-8-HQ, CTA-PEG1-8-HQ, and CTA-PEG2-8-HQ. As can be seen, all films show delectability for Zn2+ at a maximum wavelength of ca. 490 nm in the case of CTA-8-HQ, and a non-structured fluorescence in the case of CTA-PEG1-8-HQ and CTA-PEG2-8-HQ, although with higher intensity. This result also confirms the usefulness of blending PEG 600 with CTA as a good matrix for the immobilization of 8-HQ.
The detection of thallium (I) is interesting as thallium is more toxic to humans than mercury, cadmium, lead, copper, or zinc. Its chemical behavior resembles that of the heavy metal lead and the alkali metals (potassium, rubidium, cesium); and it occurs almost exclusively in natural waters as monovalent thallium (Tl+1). The solubility of thallous (Tl+1) compounds (e.g., thallous hydroxide) is relatively high so that Tl+1 is readily transported through aqueous routes into the environment. The major sources of thallium are the base metal sulfides and precious metal-bearing sulfides. Therefore, it has been shown to be a frequent contaminant in waters emanating from heavy metal deposits (e.g., sulfide-bearing deposits) [32]. Fig. 7 shows a comparative fluorescence detection of Tl+1 using CTA-8-HQ, CTA-PEG1-8-HQ, and CTA-PEG2-8-HQ. As can be seen, all films show good delectability for Tl+1 (compare with Figs. 5 and 6) at the maximum wavelength of ca. 488 nm with enhanced detectability using CTA-PEG2-8-HQ, indicating the suitability of this film for the detection of thallium (I). It is worth to mention that in spite of the water solubility of PEG 600, its leaching in aqueous solution during the fluorescence measurements was not observed. This apparent stability might be attributed to the intimate bounding of PEG inside the matrix by virtue of physical bonds such as hydrogen bonds and van der Waal interactions as well as to the fast response of the film (less than 5 min).
4 Conclusion
This study has enlightened the preparation of different optical sensors based on CTA blended with PEG 600 at two different contents and using 8-HQ as the chromophore. These films were made by the solution casting method and were characterized by FTIR and TGA. Three other films were made after solution blending with a constant concentration of 8-HQ. Fast response was observed (less than 5 min) for these sensors for the detection of different metal ions. CTA-PEG2-8-HQ shows a better detectability among all films with enhanced response for thallium (I). This result suggests a future work for the analytical quantification of thallium (I) and other heavy metal ions in aqueous solution using CTA-PEG2-8-HQ as a sensor.
Acknowledgements
This project was funded by SABIC and the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant No. MS-13-203-432. The author, therefore, acknowledges thankfully technical and financial support from SABIC and DSR.


