1 Introduction
The homogeneous catalytic oxidation of hydrocarbons under mild conditions continues to be a challenge in catalysis. Particularly, it is difficult to achieve high efficiency in the selective catalytic epoxidation of olefins with H2O2. Macrocyclic complexes of biological importance have been extensively investigated as catalysts for mild oxidation. Other oxidants such as urea hydrogen peroxide [1] or oxone [2] instead of molecular oxygen [3] are the reported systems usually employed for the epoxidations of cyclic olefins.
One of the challenging issues for chemists in the present times is the pursuit of ‘clean’ or ‘green’ chemical transformations [4]. It is often taken for granted that the use of volatile molecular solvents is a particular source of chemical waste [5,6]. Ambient-room-temperature ionic liquids have recently gained recognition as environmentally benign solvents due to their unique physical properties such as non-volatility, non-flammability, and thermal stability [7–9]. They have been employed as solvents for liquid–liquid separations, extractions and for recycling homogeneous catalysts, etc. [10–12] Ionic liquids have also been used as novel reaction media for economically and environmentally attractive processes in aqueous mono- and biphasic systems [13–17].
We had previously investigated the catalytic oxidation of primary and secondary alcohols with the same catalyst in an ionic liquid [15]. Hence, we started working towards the oxidations of other organic compounds. The present research work is one of them. Herein, we report the oxidation of selected olefins with H2O2 catalysed by cobalt-salen-triphenylphosphine complex, 1, in ionic liquid 2, under different reaction conditions. The complex was synthesized according to a reported procedure [15]. In this work, the advantageous properties of the cobalt-salen-triphenylphosphine–H2O2 oxidation system and [Emim]PF6 have been combined to give an exceptionally clean environment for catalytic oxidations. One major advantage of this system is that both the peroxide and the catalyst are soluble in the ionic liquid. This gives an oxidation solution that is completely homogeneous.
2 Experimental
2.1 General procedure for the catalytic reaction
In a typical reaction, the catalyst (8 mg, 2.4 mmol) was dissolved in [Emim]PF6 (1 cm3). After addition of the substrate (1 mmol), hydrogen peroxide (30% in water) (2 mmol) was added. The reaction mixture was stirred at room temperature for about 1 h. The completion of the reaction was monitored by GC–MS until the reactants have disappeared. After completion of the reaction, the reaction mixture was extracted with diethyl ether (3 × 3 cm3). The ether layer was collected and then a definite amount of toluene was added as an internal standard for GC analysis. The ionic liquid phase including the catalyst was washed with diethyl ether and dried prior to recycling. The epoxidation of the other olefins was carried out similarly with a little variation in reaction time (Table 2).
Epoxidation of cyclic alkenes catalyzed by complex 1a.
| Entry | Substrate | Time (h) | Conversion (%) | Selectivity (%) | |
| Epoxideb | Others | ||||
| 1 | 1 | 95.6 | 87.6 | 12.4 | |
| 2 | 1 | 92.2 | 89.2 | 10.8 | |
| 3 | 1 | 95.4 | 31.2 | 68.8c | |
| 4 | 2.5 | 94.1 | 92 | 8d | |
| 5 | 1 | 99.1 | 78.8 | 21.2e | |
| 6 | 1 | 95.6 | 82.1 | 17.9 | |
| 7 | 1 | 96.7 | 87.6 | 12.4 | |
| 8 | 1 | 96.5 | 86.4 | 13.6 | |
| 9 | 2 | 94.6 | 86.7 | 13.3f | |
| 10 | 2.5 | 97.1 | 90.2 | 9.8 | |
| 11 | 36 | 12 | 92.8 | 7.2 |
a All the reactions were carried out at room temperature.
b Epoxides were identified by using authentic samples for comparison.
c The major product was a diol.
d The major products were in the form of epoxyhexanol and epoxyhexanone.
e The minor product was aldehyde.
f The minor product was a monoepoxide.
3 Results and discussion
The epoxidation of styrene with hydrogen peroxide catalyzed by 1 was carried out in 2, affording, under stirring in the presence of hydrogen peroxide, styrene epoxide (3) and phenylacetaldehyde (4) in 78.8% and 21.2% yields, respectively. The oxidation of cyclohexene (5) with hydrogen peroxide and 1 in 2 gave epoxycyclohexane (6), 2-cyclohexenol (7) and 2-cyclohexenone (8) in 87.6, 7.1, 5.3% yields, respectively (Scheme 1), but cyclooctene gave only epoxycyclooctane in 86% yield.
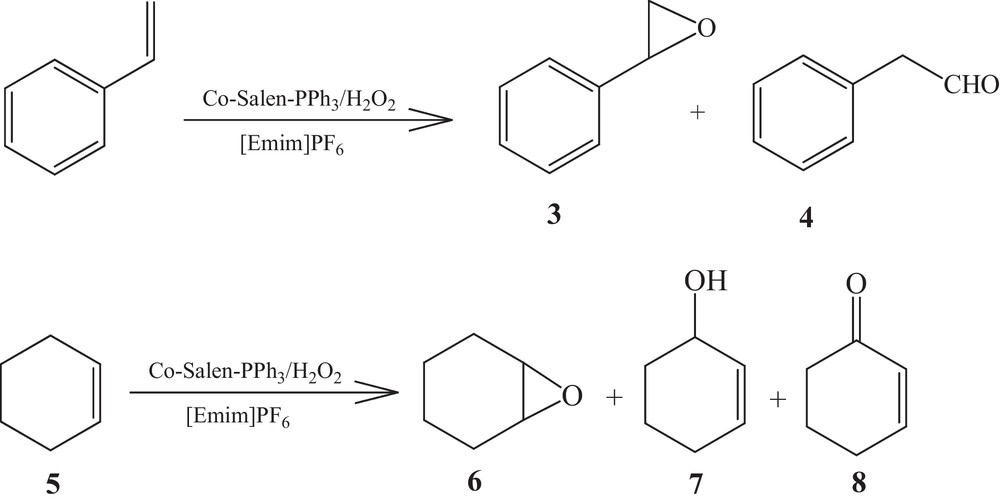
Epoxidation of alkenes by hydrogen peroxide catalyzed by 1 immobilized in 2.
Styrene was used for the optimization of the reaction conditions in ionic liquid (Table 1). The initial study was carried out using styrene as the substrate and 2 mmol of aqueous H2O2 at room temperature in the presence of 2.4 mmol of catalyst. The oxidation proceeded smoothly, and a 90% isolated yield of benzaldehyde was obtained with 99% conversion and 79% selectivity after 1-h stirring.
Epoxidation of styrenea in [Emim]PF6–H2O2 system.
| Entry | Reaction time (min) | % Conversion |
| 1 | 60 | 99.0 |
| 2b | 360 | <10 |
| 3c | 360 | NRd |
| 4e | 120 | 29.9 |
| 5f | 120 | 72.6 |
a Unless otherwise indicated, all reactions were carried out with 1 mmol of styrene with 2.4 mmol of catalyst, 2 mmol of H2O2.
b The reaction was carried out under oxygen instead of H2O2.
c Reaction without catalyst.
d NR: no reaction.
e 1.5 mmol of catalyst.
f 1 mmol of H2O2.
The oxidation occurred only in poor yield by simply bubbling molecular oxygen through the reaction mixture under similar reaction conditions (Table 1, entry 2). To evaluate the catalytic effect of the catalyst, the oxidation of styrene was carried out under similar reaction conditions in the absence of catalyst and no conversion was observed (Table 1, entry 3). The reaction was complete after 60 min at room temperature (Table 1, entry 1). Furthermore, when the oxidation was carried out using a lesser amount of the catalyst, the yield was low (Table 1, entry 5). Also the reaction was carried out by varying the oxidant's concentration to get the optimum yield (Table 1, entry 6). The organic phase containing the product was separated after the completion of the reaction, leaving behind the catalyst immobilized in the ionic liquid. The recovered brown oily ionic liquid containing 1 could be reused for further catalytic reactions (Table 2).
The results in Table 2 show that different olefinic substrates have been oxidized to epoxides, with good yields. In entry 3, to remove water completely from the system, molecular sieves were used for the reaction, and hence only the epoxide was obtained. One interesting fact is the poor conversion of 1-decene (entry 11). This may be due to the fact that 1-decene was almost insoluble in the ionic liquid and hence heterogeneity was present in the solution. Moreover, after 36 h, we observed that the reaction mixture was still intensely yellow–brown, indicating that the catalyst was still active. Also the reaction mixture was stable in ionic media. The selectivity and the yield was more and also the time required for the reaction is quite less comparable to that required for previously reported results [18–21]. The catalytic activity was particularly compared with that of the methyltrioxorehnium catalyst [22], and it was found that most of the reactions were at least comparable if not higher than the reported one. Further investigations on the catalytic activity in different ionic liquids, their kinetics and the mechanism are ongoing.
Two equivalents of hydrogen peroxide were used relative to the substrate. Remaining reactants and products were both easily removed from the reaction mixture via extraction with diethyl ether, which is immiscible with the ionic liquid used. This method of removing reactants and products is also advantageous, because the catalyst and the ionic liquid are insoluble in diethyl ether. Careful evaporation of the ether extracts gave the reactant–product mixture, which was then analyzed by GC–MS.
It is noteworthy that the recycled cobalt-salen-PPh3 catalyst 1 in ionic liquid 2 showed comparable activity, even after 6–7 runs, in the oxidation of styrene with hydrogen peroxide (Fig. 1). However, the addition of excess oxidant to the reaction mixture resulted in the degradation of the catalyst.
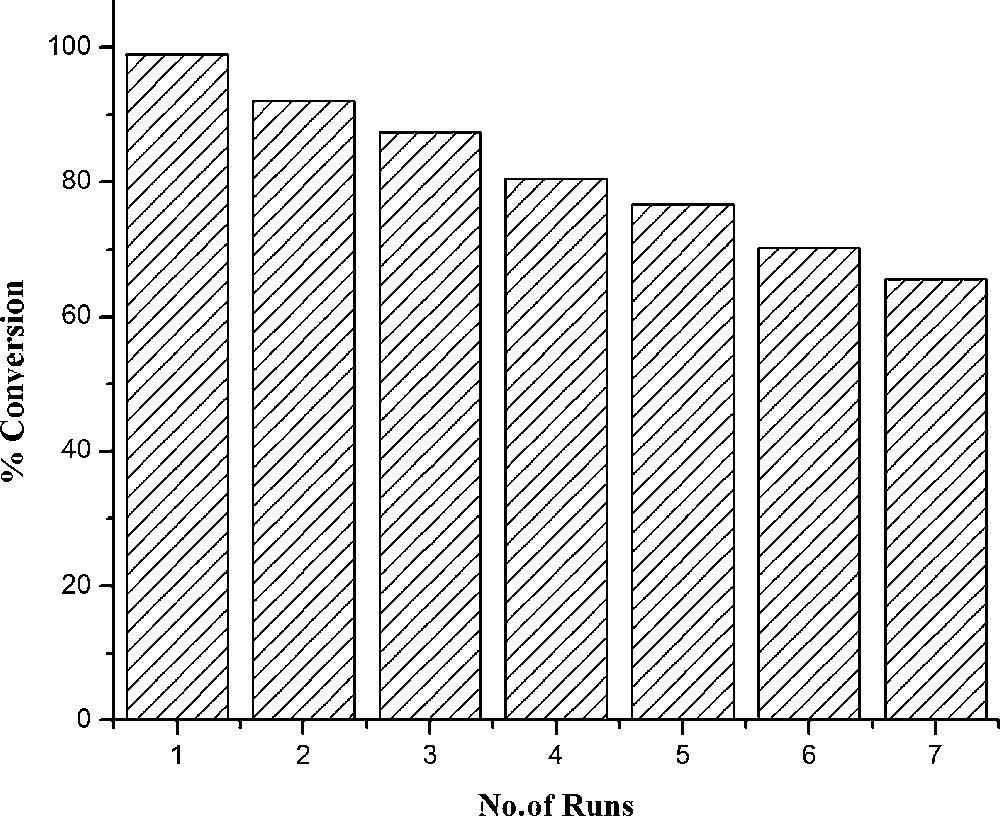
Effect of recycling on conversion.
4 Conclusion
In summary, the advantages of this oxidation system are:
- • hydrogen peroxide and catalyst are completely soluble in [Emim]PF6, giving a homogeneous oxidation solution;
- • the products are easily separated from the reaction solution by extraction with an immiscible solvent.
We also hope to expand the use of this oxidation solution to substrates other than olefins, such as hydrocarbons, amines, sulphides and other aromatic compounds.


