1 Introduction
Ortho-quinodimethanes are reactive species that are not isolable, but have been suspected as transients in 1959 by Cava et al. [1]. They were originally obtained by the double elimination of α,α′-dibromo-ortho-xylenes [1] or by thermal opening of the corresponding benzocyclobutenes [2,3], and several reviews emphasised the importance of these transients as tools for the synthesis of cyclic organic compounds [4–9]. An interesting reaction was the direct formation of the naphthalene derivatives 2 by Diels–Alder reaction with dibromo-ortho-xylylene 1 (generated from tetrabromoxylene) with spontaneous loss of HBr [1,10,11] (Scheme 1).

An early study, presented in Scheme 2, showed that the thermal isomerisation of anthracene endoperoxide 3 led to ortho-quinodimethane 5 through diepoxide 4 and finally to two successive dimers of 5, an original [8π+6π] one and the normal [8π+2π] one [12a]. Both isomeric transients 4 and 5 could be trapped with maleic anhydride (6a) or N-methylmaleimide (6b) to give the isolated endo adducts 7b and 8a,b, respectively [12b]. From these latter adducts, a base-catalysed or thermal aromatisation into the corresponding naphthalene compounds 9a and 9b has been observed, with the release of catechol as a by-product [12b].
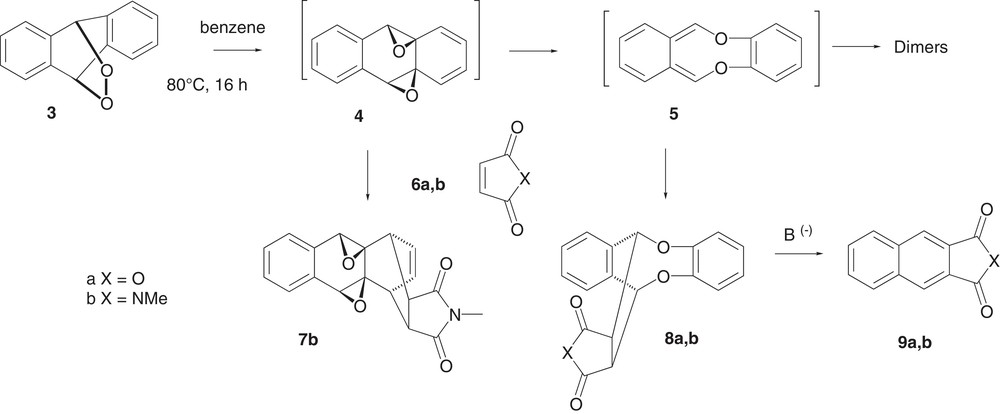
This synthesis of naphthalene compounds used only thermal and base-induced transformations and was also suitable to synthetize acid-sensitive compounds. The use of this ortho-quinodimethane 5 in organic synthesis has never been studied, although the latter was very easy to obtain from the cheap anthracene. The purpose of this work was to investigate the scope of this Diels–Alder reaction and the further formation of naphthalene derivatives.
2 Results and discussion
2.1 Case of reactive dienophiles
The addition of N-methylmaleimide 6a or maleic anhydride 6b has already been studied in the initial study of the thermal isomerisation of anthracene epidioxide 3 in refluxing benzene. The corresponding adducts 7b (10%) and 8a,b (70–76%) have been isolated along with minute amounts of anthraquinone [12b].
In the present work (Scheme 3), the thermal isomerisation of epidioxide 3 was performed at higher temperature in order to favour the reaction with ortho-quinodimethane 5, i.e. in refluxing chlorobenzene (ca. 132 °C) for 1 h in the presence of a dienophile in slight excess (ca 1.2 equiv.).

Using 1,4-benzoquinone 10a, 1,4-naphthoquinone 10b or trans-dibenzoylethylene 11 as dienophiles, adducts 14a, 14b or 15, respectively, were isolated in good yields (60–70%).
In the particular case of benzoquinone 10a, a double addition was observed when epidioxide 3 was used in two-fold excess. However, the adduct could not be isolated as a pure compound and was treated directly with a base (see below).
2.2 Case of less reactive dienophiles
Cyclohex-2-en-1-one, cyclohexene, and coumarin were unreactive and dimers of 5 were only observed. In the case of the cyclopentenone 12, the adduct 16 was isolated in poor yield (20–30%) and required to heat under refluxing benzene for 16 h with an 8-fold excess of the dienophile (Scheme 3). An inactivated double bond or a double bond activated by only one carbonyl function was also too less reactive for this Diels–Alder reaction to compete with dimerization. The more reactive 5-membered ring 12 gave only poorly the desired adduct.
2.3 Structure of the adducts
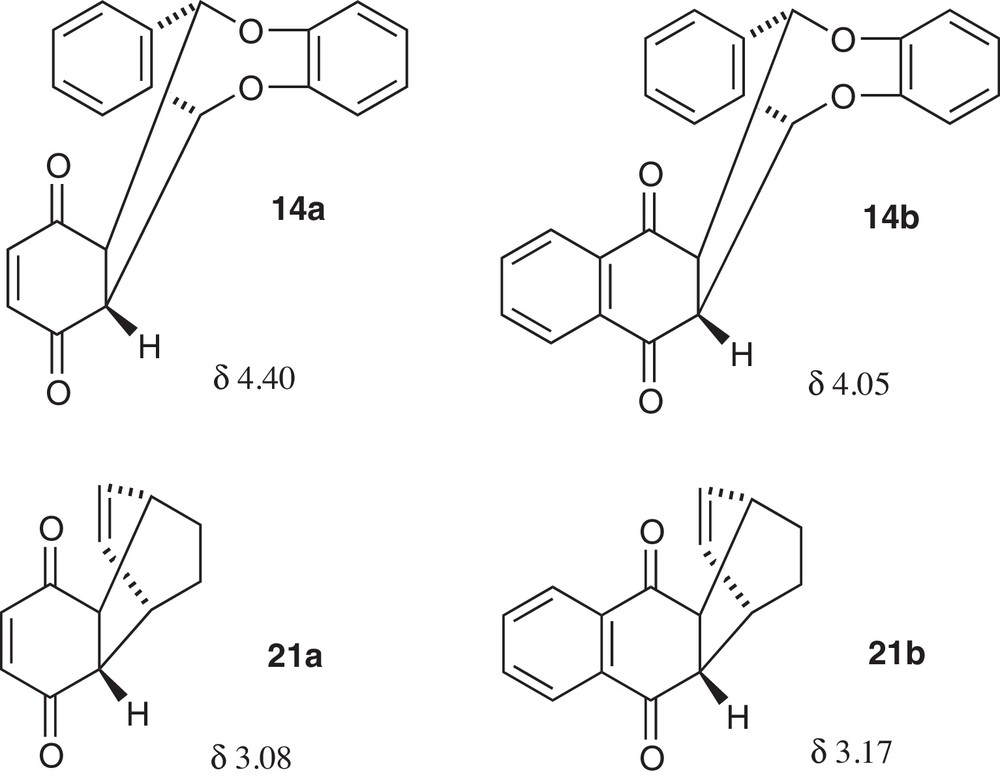
In all cases, we obtained a single adduct. Adduct 15 possessed the benzoyl groups in trans relation, as indicated by the important coupling between the protons in α-position to the carbonyl functions (J = 8 Hz). We assumed that adducts 14a,b and 16 possessed an endo-configuration (see Scheme 3) as it has already been demonstrated for 8b [12b]. This was confirmed in 1H NMR spectra of 14a and 14b by the clear deshielding of the protons H-α in α-position to the carbonyl groups (δ 4.40 and 4.05 ppm, respectively) in comparison to the corresponding protons of the similar adducts 21a [13] and 21b [14] of the quinones 10a and 10b with cyclohexadiene (δ 3.22 and 3.17, respectively), as depicted in Scheme 4. This deshielding was due to the influence of the nearby intracyclic oxygen atoms of the veratrole moiety. In the case of the non-symmetrical adduct 15, this important effect shifted one of the H-α protons at 5.90 ppm, with also a deshielding of 1.4 ppm with respect to the other H-α (at 4.50 ppm).
2.4 Base-induced cleavage of the adducts
Instead of an aqueous sodium carbonate solution under refluxing THF as originally described [12b], we have preferred to use the triethylamine as a more suitable and efficient base (Scheme 3). For mono-adducts 14a and 14b, the addition of an excess of triethylamine at room temperature was sufficient to cleave these adducts into 1,4-anthraquinone 18a or naphthacene-5,12-quinone 18b, which were isolated in good yields by simple crystallisation and washing in order to discard the formed catechol. It is worth noting that this transformation could be carried out in a one-pot process. For instance, endoperoxide 3 was refluxed with a slight excess of benzoquinone 10a for 1 h. The solvent was then partially evaporated, and after addition of an excess of triethylamine, quinone 18a was isolated. However, using half the amount of 10a led to a double addition of ortho-quinodimethane 5 onto this benzoquinone, followed by a double cleavage after the addition of triethylamine to give pentacenequinone 17. In both the cases, the overall yield of quinones 18a or 17 was similar (ca 65%). Concerning adducts 15 and 16, their cleavage with triethylamine was more difficult and was achieved under refluxing 1-propanol for one day to give the known 2,3-dibenzoylnaphthalene 19 [15] and benzo[f]indanone 20 [11] in 68% yield, respectively. In these cases, the catechol by-product was discarded by oxidation of the crude product on silica gel plates (see Experimental part).
Actually, the dibromo-ortho-quinodimethane transient 1 was more reactive than the ortho-quinodimethane 5 intermediate. This was exemplified with benzoindenone 20 [11], which was obtained in better yield with dibromo 1. However, the formation of reactive hydrobromic acid limited its use, even if the addition of calcium carbonate improved the conditions [16].
3 Conclusion
We have studied the scope of the Diels–Alder reaction of the transient phenylenedioxy-ortho-quinodimethane 5, issued from the thermal isomerisation of anthracene epidioxide 3. We have then developed a very mild preparation of naphthalene derivatives by triethylamine-catalysed cleavage of the corresponding adducts. This method allows the synthesis of acid-sensitive compounds and offers certain advantages over the classical method using dibromo-ortho-quinodimethane 1, but seems limited to reactive dienophiles as quinones 10a,b or dibenzoylethylene 11.
4 Experimental part
4.1 General
Flash chromatography (FC): silica gel (Merck 60, 230–400 mesh). TLC: Al-roll silica gel (Merck 60, F254). Mp: Kofler hot bench. IR spectra (ν in cm−1): PerkinElmer 297. UV spectra: Spectrometer Cary 15. 1H and 13C NMR spectra (80 MHz and 20 MHz resp.): Varian FT 80A, tetramethylsilane (TMS) as an internal standard. Microanalyses were carried out by the Laboratoire de microanalyses, Université Paris-6, France.
4.2 Reagents and solvents
Usual solvents and PhCl were freshly distilled, dry Et2O and benzene were distilled and stored over Na, CHCl3 was distilled over P2O5 and kept over Na2CO3.
4.2.1 Preparation of the 9,10-epidioxy-9,10-dihydroxyanthracene (3) [12b]
To a solution of anthracene (4.0 g, 22 mmol) in CHCl3 (600 mL) was added a solution of hematoporphyrin hydrochloride (50 mg) in EtOH (100 mL) and this solution was irradiated with a halogen lamp whose light was filtered through an aqueous solution of Na2CrO4 (20 g/L). The anthracene disappearing was monitored by UV-spectroscopy or TLC. The dye was discarded by the addition of solid Na2CO3 and filtration. The solution was then evaporated, the residue crystallised and washed in EtOH and Et2O to give cream crystals of 3 (4.2 g, 84%).
4.3 Preparation of the adducts
4.3.1 General procedure
A solution of 3 (0.2 g, 0.95 mmol) and the dienophile (ca 1.2 equiv.) in chlorobenzene (20 mL) was heated under reflux for 1 h. The solvent was then evaporated and the adduct isolated by crystallisation and washing in the given solvent.
4.3.2 Benzoquinone adduct 14a: general procedure with 3 (0.3 g, 1.43 mmol) and 10a (0.19 g, 1.76 mmol, 1.23 equiv.)
Cream crystals from acetone, 60% yield, mp (dec) > 290 °C (C6H5Cl).
IR (KBr): 1670 (CO), 1485, 1240, 810, 760 cm−1. UV (THF), λmax(log ɛ): 385 (1.78); 283 (3.19); 270 (3.26); 262 (3.25) nm. 1H NMR (CDCl3): δ 4.40 (m, 2 H, 2 H-α); 5.80 (m, 2 H, H-6, H-11); 6.80 (s, 4 H, H-1 to H-4); 7.20 (s, 6 H, 2 H-β, H-7 to H-10). 13C NMR (CDCl3): δ 52.1 (2 C-α); 81.1 (C-6, C-11); 123.2, 124.6 (C-1 to C-4); 129.6, 130.4 (C-7 to C-11); 133.5 (C-6a, C-10a); 141.1 (2 C-β); 149.0 (C-4a, C-12a); 195.7 (2 CO) ppm. Anal. calcd for C20H14O4 (318.0): C, 75.46; H, 4.43; O, 20.10. Found: C, 75.7; H, 4.6; O, 20.2.
4.3.3 Naphthalenequinone adduct 14b: general procedure with 3 (0.2 g, 0.95 mmol) and 10b (0.20 g, 1.28 mmol, 1.35 equiv.)
Cream crystals, 65–70% yield, mp 271 °C (benzene).
IR (KBr): 1670 (CO), 1240, 980, 950, 800, 745 cm−1. UV (THF), λmax(log ɛ): 385 (2.26); 295 (3.39); 278 (3.51) nm. 1H NMR (CDCl3): δ 4.05 (m, 2 H, 2 H-α); 6.00 (m, 2 H, H-6, H-11); 6.80 (s, 4 H, H-1 to H-4); 7.20 (s, 4 H, H-7 to H-10); 7.50–8.00 (m, 4 H, 2 H-γ, 2 H-δ). 13C NMR (CDCl3): δ 52.8 (2 C-α); 81.2 (C-6, C-11); 123.3, 124.5 (C-1 to C-4); 127.0 (2 C-β); 129.7, 130.2 (C-7 to C-11); 133.9 (C-6a, C-10a); 134.6, 134.8 (2 C-γ, 2 C-δ); 149.0 (C-4a, C-12a); 195.2 (2 CO) ppm. Anal. calcd for C24H16O4 (368.4): C, 78.25; H, 4.38; O, 17.37. Found: C, 78.3; H, 4.4; O, 17.2.
4.3.4 trans-2,3-Dibenzoylethylene adduct 15: general procedure with 3 (0.5 g, 2.4 mmol) and 11 (0.65 g, 2.75 mmol, 1.16 equiv.)
Cream crystals from boiling cyclohexane, 65% yield, mp 210–211 °C. IR (KBr): 1670 (CO), 1490, 1440 cm−1. UV (THF), λmax(log ɛ): 316 (2.38); 277.5 (3.55); 272.5 (3.55); 244 (4.50) nm. 1H NMR (CDCl3): δ 4.50 (dd, J = 1.7, 8.2 Hz, 1H, H-α); 5.75 (d, J = 0.9 Hz, 1 H, H-11); 5.79 (d, J = 1.7 Hz, 1 H, H-6); 5.90 (dd, J = 0.9, 8.2 Hz, 1 H, H-α′); 6.73 (s, 4 H, H-1 to H-4); 7.21 (s, 4 H, H-7 to H-10); 7.30–7.70 (m, 6 Har); 7.90-8.10 (m, 4 Har). 13C NMR (CDCl3, partial data without aromatic C between 125–139 ppm): δ 49.0, 53.3 (C-α, C-α′); 80.2, 82.2 (C-6, C-11); 148.9, 150.7 (C-4a, C-12a); 195.6, 196.2 (2 CO) ppm. Anal. Calcd for C30H22O4 (446.5): C, 80.70; H, 4.97; O, 14.33. Found: C, 80.5; H, 5.0; O, 14.3.
4.3.5 Cyclopentenone adduct 16
A solution of 3 (0.5 g, 2.4 mmol) and distilled 12 (1.6 mL, 19.2 mmol, 8.1 equiv.) in benzene (250 mL) was refluxed for 16 h. The solvent was evaporated to give a mixture of adduct 16 and 9,10-anthraquinone. After purification by column chromatography (SiO2, eluent CH2Cl2), 16 was isolated (0.15–0.2 g, 20–30% yield).
Cream crystals, mp 228–229 °C (benzene). IR (KBr): 1740, 1485, 1240, 770 cm−1. 1H NMR (CDCl3): δ 1.80–2.80 (m, 4 H); 3.52, 3.60 (2 m, each 1 H, H-α, H-α′); 5.32, 5.62 (2 m, each 1 H, H-6, H-11); 6.76 (s, 4 H, H-1 to H-4); 7.21 (s, 4 H, H-7 to H-10). Anal. calcd for C19H16O3 (292.2): C, 78.06; H, 5.52. Found: C, 77.9; H, 5.7.
4.4 Cleavage of the adducts
4.4.1 Pentacene-6,13-quinone (17)
4.4.1.1 From anthracene epidioxide 3
A solution of 3 (0.1 g, 0.47 mmol), 10a (24 mg, 0.22 mmol, 0.47 equiv.) was refluxed in PhCl (25 mL) for 1 h. The solvent was evaporated until 5–10 mL, and NEt3 (0.5 mL) was added at room temperature. The precipitated yellow crystals of 17 (40 mg, 63%) were isolated after 1 h of stirring. Mp 394 °C (lit. [1] 395–398 °C). Same IR and 1H NMR data as in the literature [17].
4.4.2 1,4-Anthraquinone (18a)
4.4.2.1 From adduct 14a
To a solution of adduct 14a (0.22 g, 0.68 mmol) in CHCl3 (5 mL) was added NEt3 (0.5 mL) at room temperature and the solution was stirred for 1 h. The solution was evaporated and the yellow plates of 18a (135 mg, 95%) were washed with Et2O.
4.4.2.2 From anthracene epidioxide 3
Same procedure as for 17 with 3 (0.2 g, 0.95 mmol), 10a (0.15 g, 1.39 mmol, 1.46 equiv.) in PhCl (20 mL), to give 18a (130 mg, 65%) as yellow plates. Mp 225 °C (lit. [1] 219–223 °C). Same IR, 1H NMR and 13C NMR data as in the literature [17].
4.4.3 Naphthacene-5,12-quinone (18b)
Same procedure as for 18a, from adduct 14b (0.25 g, 0.68 mmol) in CHCl3 (5 mL) and NEt3 (0.5 mL) to give 18b (165 mg, 95%) as yellowish crystals. Mp > 280 °C (lit. [1] 290–292 °C). Same IR data as in the literature [17].
4.4.4 2,3-Dibenzoylnaphthalene (19)
A solution of 15 (0.13 g, 0.29 mmol) in propan-1-ol (35 mL) was refluxed with NEt3 (5 mL) for 24 h. The solvents were evaporated, the residue dissolved in CHCl3 (20 mL) and silica gel (Kieselgel 60, 4 g) was added. This mixture was left to dry for 16 h in order to oxidize the formed pyrocatechol, then extracted with CH2Cl2 and the solvent evaporated to give 19 (66 mg, 68%) as colourless crystals after recrystallisation in AcOH. Mp 145 °C (AcOH) (lit. [15] 145 °C). Same IR data as in the literature [18].
4.4.5 Benzo[f]indanone (20)
Same procedure as for 19 to give 20 as colourless crystals (68% yield). Same 1H NMR as in lit. [11]. IR (KBr): 2940, 1705, 1630, 870 cm−1.
Acknowledgements
The support of the Centre national de la recherche scientifique (CNRS) was gratefully acknowledged. We thank also Dr Nguyen Kim Cuong and J. Baranne-Lafont for their interest in this work.


