1 Introduction
Coumarins are important because of their potential applications in pharmaceutical, fragrance and agrochemical industries [1]. Bis-coumarin derivatives have been applied as antibiotics, coagulants, antitumor drugs, HIV protease inhibitors as well as additives in food and cosmetics [2–5]. Pechmann, Perkin, Knoevenagel, Reformatsky, and Wittig reactions are some of the previous methods for the synthesis of bis-coumarins. It is noticeable that the often-used method for the preparation of bis-coumarins is the Knoevenagel reaction using different types of acid catalysts, such as sulfuric acid, phosphorus pentoxide, aluminum chloride, iodine, and trifluoroacetic acid [6–8]. However, some of them suffer from drawbacks, such as the use of toxic metals or of volatile organic solvents, high cost and low yields. Therefore, it is highly desirable to develop efficient and cost-effective catalysts and procedures with high novelty for the preparation of these valuable compounds.
Ionic liquids (ILs) have received considerable interest as eco-friendly solvents, catalysts and reagents in organic transformations, because of their unique properties such as low volatility, non-flammability, high thermal stability, negligible vapor pressure and ability to dissolve a wide range of materials [9]. In extension of our ongoing programs on the preparation and application of acidic ILs in organic synthesis, we have recently introduced a new category of ionic liquids, namely sulfonic-acid-functionalized imidazolium salts (SAFIS), and used them as efficient catalysts in some organic transformations [10–22]. Having the above facts in mind, we have recently synthesized and characterized sulfonic-acid-functionalized pyridinium chloride {[Pyridine–SO3H]Cl} as a new ionic liquid (Scheme 1), and successfully used it in some organic transformations [23–25]. In continuation, [Pyridine–SO3H]Cl was successfully utilized as a catalyst for the synthesis of bis-coumarins and 3,3′,3′′,3′′′-(1,4-phenylenebis(methanetriyl))tetrakis(4-hydroxy-2H-chromen-2-one) (Scheme 2).
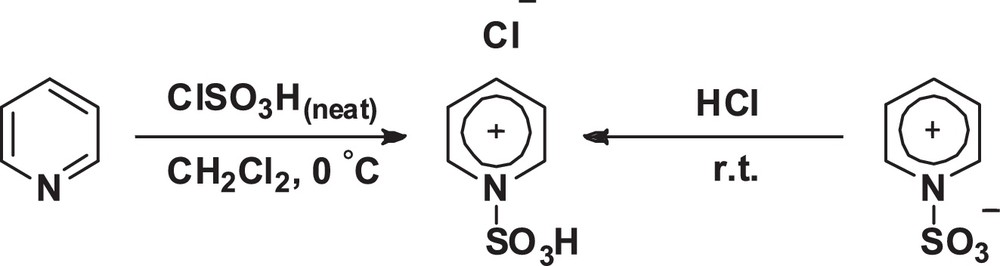
Preparation of [Pyridine–SO3H]Cl.

(Color online.) Preparation of bis-coumarin derivatives.
2 Results and discussion
Sulfonic-acid-functionalized pyridinium chloride [Pyridine–SO3H]Cl was prepared by the reaction of pyridine (1 equiv) with chlorosulfonic acid (1 equiv) in dry CH2Cl2 at 0 °C with high atomic economy. Alternatively, it was synthesized by the reaction of the sulfur trioxide pyridine complex with an excess amount of hydrogen chloride gas at room temperature (Scheme 1).
To optimize the reaction conditions, as model reaction, the solvent-free condensation of 4-hydroxycoumarin and 4-nitrobenzaldehyde was tested using different amounts of [Pyridine–SO3H]Cl in the range between 70 and 100 °C. The best results were obtained using 15 mol% of [Pyridine–SO3H]Cl at 80 °C. Increasing the reaction time did not improve the results (Table 1).
Effect of different amounts of the catalyst and temperatures on the reaction between 4-hydroxycoumarin and 4-nitrobenzaldehyde.
| Catalyst | Mol % of Catalyst | Temp. (°C) | Time (min) | Yield (%)a |
| – | – | 80 | 60 | 20 |
| [Pyridine–SO3H]Cl | 10 | 80 | 19 | 81 |
| [Pyridine–SO3H]Cl | 15 | 80 | 12 | 92 |
| [Pyridine–SO3H]Cl | 20 | 80 | 13 | 92 |
| [Pyridine–SO3H]Cl | 15 | 70 | 25 | 78 |
| [Pyridine–SO3H]Cl | 15 | 100 | 12 | 92 |
a Isolated yield.
To explore the generality and scope of the catalyst, we extended our study using [Pyridine–SO3H]Cl (15 mol %) in the absence of solvent with different aromatic aldehydes to prepare a series of bis-coumarin derivatives (Table 2). Various aromatic aldehydes containing electron-withdrawing substituents, electron-releasing substituents and halogens on their aromatic rings as well as heteroaromatic aldehydes were utilized successfully in the reaction, and gave the corresponding products in high yields and in short reaction times. Moreover, the condensation of 4-hydroxycoumarin (2 equiv) with terephthaldehyde (1 equiv) in the presence of [Pyridine–SO3H]Cl (30 mol%) at 80 °C under solvent-free conditions afforded 3,3′,3′′,3′′′-(1,4-phenylene bis (methanetriyl)) tetrakis(4-hydroxy-2H-chromen-2-one) in 74% yield within 24 min (Scheme 2). Thus, the catalyst was general and highly efficient.
Synthesis of bis-coumarins using [Pyridine–SO3H]Cl (15 mol %) under solvent-free conditions at 80 °C.
| Entry | Product | Time (min) | Yield (%)a | Mp. °C (Lit.) |
| 1 | 14 | 88 | 230–232 (230–232) [26] | |
| 2 | 12 | 92 | 259–261 (256–258) [26] | |
| 3 | 14 | 89 | 260–262 (260–262) [27] | |
| 4 | 12 | 93 | 232–234 (232–234) [26] | |
| 5 | 13 | 91 | 205–207 (200–202) [28] | |
| 6 | 15 | 84 | 270–274 | |
| 7 | 16 | 81 | 197–201 | |
| 8 | 20 | 87 | 188–191 (190) [29] | |
| 9 | 21 | 80 | 132–136 (133) [29] |
a Isolated yield.
In a mechanism that is proposed in Scheme 3, at first, 4-hydroxycoumarin reacts with the carbonyl group of the aldehyde, which is activated by [pyridine–SO3H]Cl via hydrogen bonds, and affords intermediate I. Then, II is prepared by dehydration reaction. In the next step, II acts as a Michael acceptor and is activated by the catalyst to react with another molecule of 4-hydroxycoumarin to give III. Finally, III is converted into IV after tautomerisation as the main product.
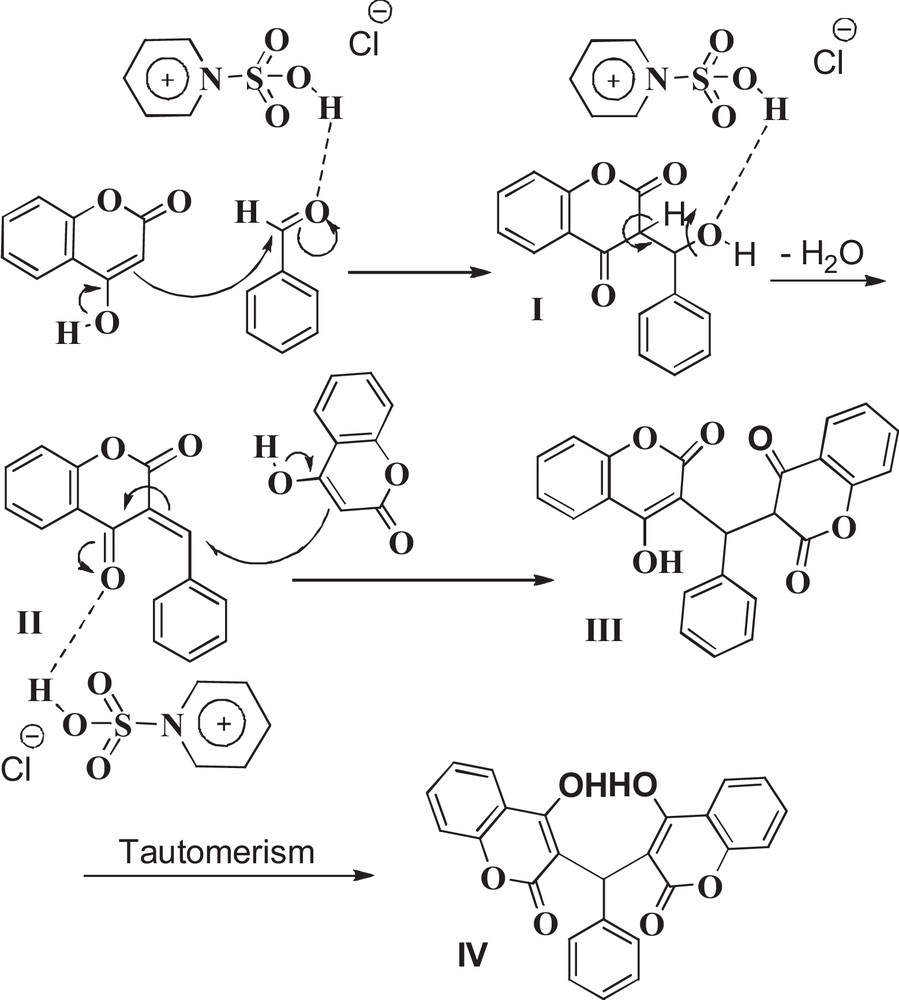
Proposed mechanism for the synthesis of bis-coumarins.
In another investigation, the reusability of the catalyst was tested upon the reaction of 4-hydroxycoumarin (2 mmol) and 4-chlorobenzaldehyde (1 mmol). The reaction mixture was extracted by a warm mixture of ethyl acetate and chloroform (7:3) to separate the products from the catalyst. Afterward, the reused catalyst was employed for another reaction. We observed that the catalytic activity of the catalyst was restored within the limits of the experimental errors after three successive runs (Fig. 1).
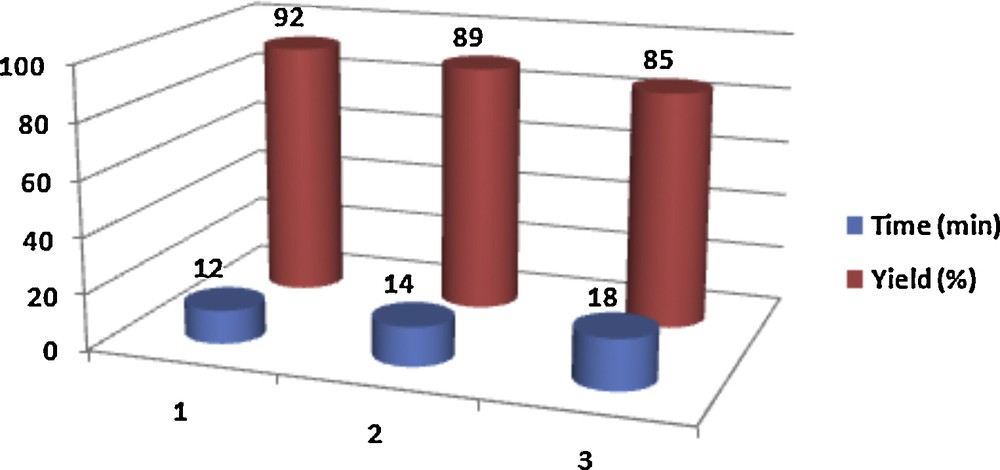
(Color online.) Synthesis of bis-coumarins in the presence of reused [Pyridine–SO3H]Cl under solvent-free conditions at 80 °C.
3 Conclusion
In conclusion, we have introduced a new and highly efficient ionic liquid, namely sulfonic-acid-functionalized pyridinium chloride [Pyridine–SO3H]Cl, as a reusable catalyst for the synthesis of bis-coumarin derivatives and 3,3′,3′′,3′′′-(1,4-phenylenebis(methanetriyl))tetrakis(4-hydroxy-2H-chromen-2-one) as potentially biologically interesting compounds.
4 Experimental
4.1 General
All chemicals were purchased from Merck, Fluka or Acros Chemical Companies and used without any further purification. The products were identified by comparison of their 1H NMR, 13C NMR IR spectrum and TLC with those of authentic samples. The progress of the reactions was monitored by TLC using silica gel SIL G/UV 254 plates. The 1H NMR (400 MHz) and 13C NMR (100 MHz) were run on a Bruker Avance DPX FT-NMR spectrometer (δ in ppm). Melting points were recorded on a Büchi B-545 apparatus in open capillary tubes.
The infrared spectra of the compounds were recorded using a PerkinElmer PE-1600-FTIR device.
4.2 General procedure for the synthesis of bis-coumarin using [Pyridine–SO3H]Cl
A mixture of 4-hydroxycoumarin (0.324 g, 2 mmol), aldehyde (1 mmol) and [Pyridine–SO3H]Cl (0.0292 g, 15 mol%) was added to a test tube, and stirred at 80 °C. After completion of the reaction, which was monitored by TLC, the reaction mixture was cooled to room temperature, extracted with a warm mixture of ethyl acetate and chloroform (7:3) (20 mL) to separate the catalyst. The crude products were soluble in a warm mixture of ethyl acetate and chloroform (7:3), and the catalyst was insoluble. The remaining viscous liquid was washed with ethyl acetate (5 mL) and dried under reduced pressure in order to be used for the next run. Then, ethyl acetate was evaporated and the solid residue (crude product) was triturated by a mixture of ethanol and water (9/1) to give the pure product.
Acknowledgements
The authors gratefully acknowledge the Bu-Ali Sina University Research Council and Center of Excellence in Development of Environmentally Friendly Methods for Chemical Synthesis (CEDEFMCS) Hamedan, I. R. Iran.


