1 Introduction
Notes: In the text that follows, the following definitions are used.
(a) Conglomerate crystallization: a particularly thorough description of the state of scientific understanding of the phenomenon, up to 1981, is the monograph by Jacques et al. [1], who go on to say that “crystalline racemates may belong to one of three different classes. In the first, the crystalline racemate is a conglomerate, that is, a mechanical mixture of crystals of the two pure enantiomers. A conglomerate is formed as a result of a spontaneous resolution”. A remarkably modern, early description of the physicochemical aspects of the phenomenon was given by Kipping and Pope [2] as summarized by Bernal [3].
(b) Kryptoracemic crystallization: in a tour-de-force, Fabián and Pratt [4] enumerated and described all those organic compounds (181 in total) appearing in CSD1 that crystallize as kryptoracemates, a classification defined as racemic pairs crystallizing in a Sohncke space group”, who stated (ref. 4 below, p. 95) that the term was originally coined by Bernal [5–9]. An illustration of this mode of crystallization is given in Fig. 1, taken from the data in [7].
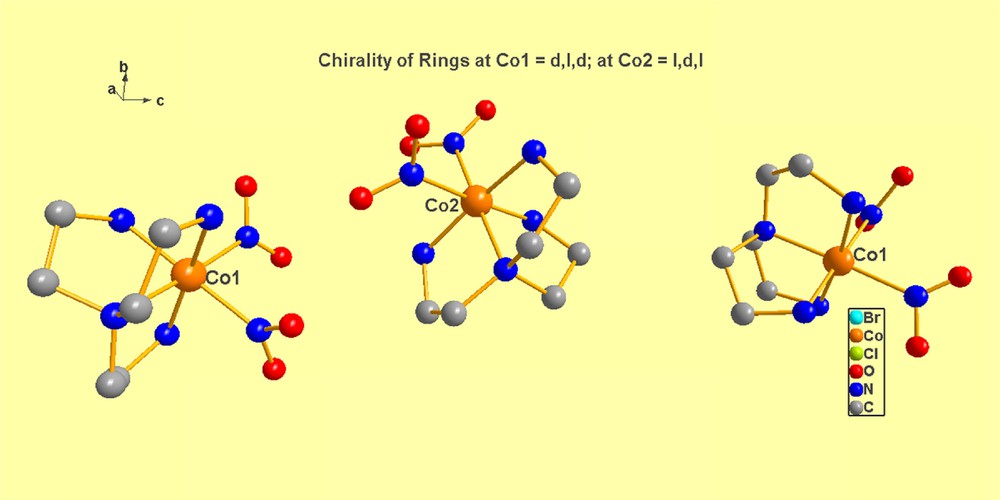
(Color online.) In NIXGIK [7], the cobalt cations align themselves in rows of cations 1, 2, 1, 2, … 1, 2 along the c-screw-axis of space group P212121, as shown above. Note that the sequence of torsional angles in Co(1) is δ, λ, δ, while that in Co(2) is λ, δ, λ. Thus, the helical sense in the two cations is opposite, as required for kryptoracemic crystallization. Note also, that the magnitude of the torsion angles is not exactly the same, as pointed out in the paper, since packing considerations preclude this, given the differences in such forces at different sites of a crystalline material, especially a non-centric one.
In what follows, the graphics were generated with program DIAMOND [10], which we found invaluable for such enterprise.
(c) Unbalanced crystallization: this term was so coined by Albano et al. [11] who stated that an Ir compound, TPNOIR, they had recently studied crystallized with three molecules in the asymmetric unit (Fig. 2, with Z′ = 3) and that two of them shared the same chirality and differed from the third one. This phenomenon was, thereby, said to be a case of “unbalanced crystallization”.
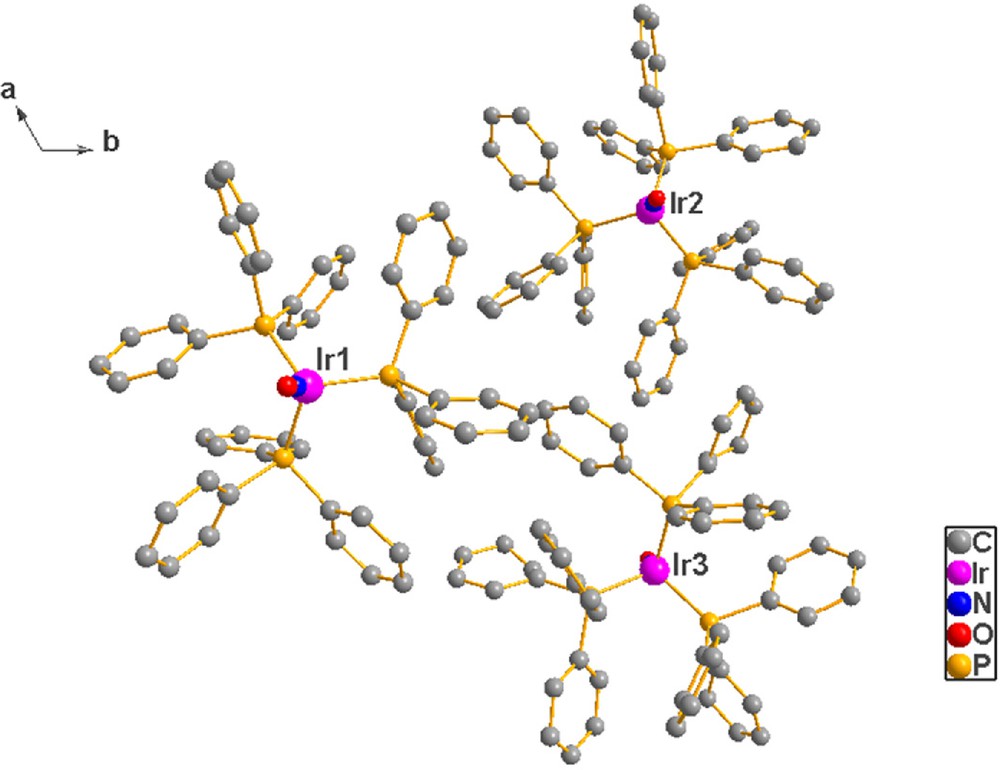
(Color online.) The three molecules present in the asymmetric unit of the TPNOIR lattice. The centroid of the atoms in the asymmetric unit is located, within standard deviations, at (0, 0, 0). Note that the propeller sense of the planes of the phenyl rings of the triphenylphosphine ligands define an anti-clockwise sense in molecules 1 and 2, while their sense in molecule 3 is clockwise; thus, the chiral unbalance discovered, and named, by Albano et al. [11].
Given the difficulties inherent in scanning CSD1, as detailed by Fabián and Brock [4], it was quite by chance that we ran into an apparently unique example of all three modes of crystallization in a single crystalline substance, namely TIQHOR [12]. Equally by chance, we discovered VAGBOU [13], which provides a case of conglomerate and kryptoracemic crystallization; and finally, searching for information on structures of Co(III) coordination compounds crystallizing in Sohncke space groups, we ran into a very intriguing compound labeled ZIFHEB [14], whose stereochemistry is remarkably simple, yet it meets all of the requirements for a conglomerate and a kryptoracemate.
2 The structure of TIQHOR
As stated earlier, TIQHOR is the compound bis(bis(ethylenediamine-N,N′)-(oxalato-O,O′)-cobalt) biphenyl-4,4′-disulfonate hydrate, whose structure was described by Wang and Sevov [12]. It crystallizes in space group P21, with four cobalt cations in the asymmetric unit. [Note that CSD says that Z′ = 2; however, since they list the formula as 2(C6H16CoN4O4+), C12H8O6S22−, 3.5(H2O), the reality is that, as far as the cations are concerned, Z′ = 4. This is one of the serious problems described by Fabián and Brock [4] in searching CSD for information on kryptoracemic crystallization; see p. 95 of their paper]. It is equally daunting when searching for information on unbalanced crystallization, as we found out (Figs. 3–7).

(Color online.) TIQHOR. The asymmetric unit contains four cobalt cations. Notice that they align themselves, approximately, along the b-screw-axis of the crystal. Not shown, is the fact that they are held together by hydrogen bonds to the sulfonate anions and the waters of crystallization, a fact that was clearly detailed by the authors of the report on the structure of this compound [12], but not displayed here to avoid cluttering due to overlap of atoms along the line of sight most convenient to display all four cations in one view.

(Color online.) Co(1) is Λ(δλ). Numerical details of the torsion angles in the two (en) ligands were provided above. Note the differences in the chiro-optical symbols in the cations shown in Figs. 4–7.

(Color online.) Co(2) Δ(λδ). Numerical details of the torsion angles in the two (en) ligands were provided above. Note the differences in the chiro-optical symbols in the cations shown in Figs. 4–7.

(Color online.) Co(3) Λ(δδ). Numerical details of the torsion angles in the two (en) ligands were provided above. Note the differences in the chiro-optical symbols in the cations shown in Figs. 4–7.
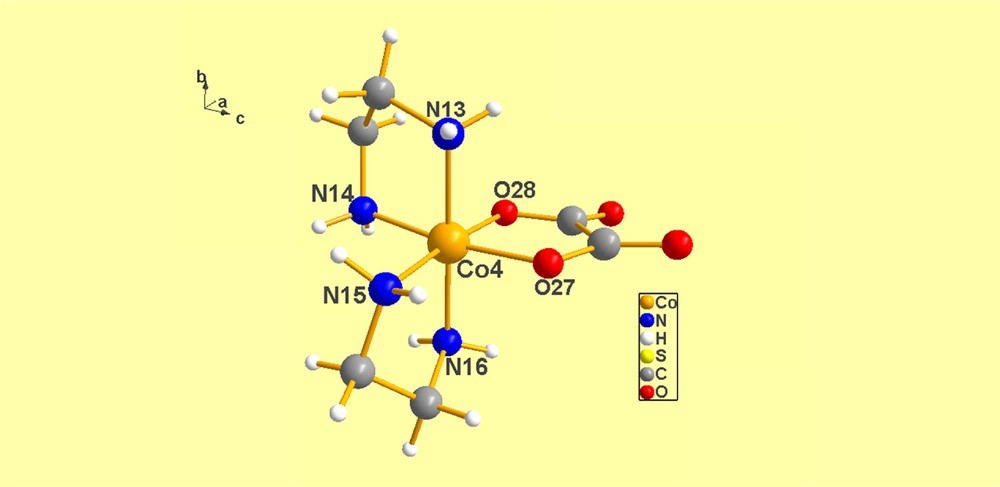
(Color online.) Co(4) Λ(λδ). Numerical details of the torsion angles in the two (en) ligands were provided above. Note the differences in the chiro-optical symbols in the cations shown in Figs. 4–7.
Chirality data (1): the ethylenediamine ligands in TIQHOR:
Co(1) Λ(δλ) N4–C27–C28–N3 = 51.44, N1–C25–C26–N2 = –50.74;
Co(2) Δ(λδ) N6–C32–C31–N5 = –51.05, N7–C36–C35–N8 = 51.27;
Co(3) Λ(δδ) N11–C41–C42–N2 = 53.03, N9–C39–C40–N10 = 49.88;
Co(4) Λ(λδ) N16–C47–C48–N15 = –53.83, N14–C46–C45–N13 = 47.03.
Chirality data (2): the O–C–C–O torsion angles on the Co side:
Co(1) = 8.51 Co(2) = –8.63 Co(3) = 8.09 Co(4) = 9.08°.
Thus, the crystal qualifies as a conglomerate since all four cations are chiral and stereochemically stable. Numerically speaking, there are two pairs [Co(1) vs. Co(2)] and [Co(1) vs. Co(4)] that constitute enantiomeric pairs; thus, the crystal qualifies as a kryptoracemate. Finally, Co(3) is different from the other ones in that it is not the enantiomer of any of the other three; therefore, the crystal is chiraly unbalanced.
As documented above, the structures of all four cations reveal that the oxalate ligands are not flat; in fact, the torsion angles defined by the (ligand oxygen–C–C–ligand oxygen fragment) follow the same pattern of being unbalanced, as was the case with the conformation of the (en) ligands to the metal. Moreover, the magnitude of the torsion is somewhat different in each case, no doubt being the result of differences in environment for each metal cation.
Interestingly, a search of CSD1 for “Any transition metal with two ethylenediamine and one oxalate ligand and Z′ ≥ 2′′ only produces one hit other than TIQHOR, see Fig. 4, and that is VAGBOU [13] which is a conglomerate as well as a kryptoracemate, but, since the real Z′ = 2, it is not unbalanced. However, its asymmetric unit is very well-suited to illustrate the packing of a cell that is both conglomerate and kryptoracemic, as displayed in Fig. 8, below.
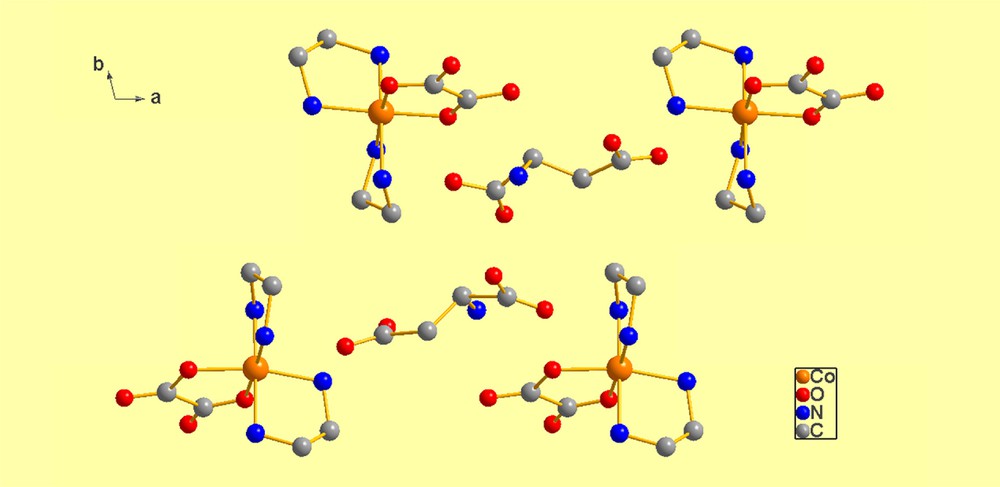
(Color online.) VAGBOU. At the center of the figure, there are two carboxylate fragments, between which there is a pseudo-inversion center about which pairs of enantiomers face each other, as is very obvious. The inversion center is imperfect because (a) the space group is a Sohncke space group and (b) the torsion angles of the ethylenediamine ligands are somewhat different in value, even if they are opposite in sign. Thus, it is not surprising that the pseudo-inversion center is located, approximately, at ½, ½, ½ (actually, at 0.4700, 0.5130, 0.5300).
ZIFHEB [14] crystallizes in space group P21, with Z = 4, and Z′ = 2. It was crystallized from a racemic solution and is chiral for two reasons: (a) upon binding the Co(III) cation, the diamine ligand nitrogen containing the phenyl ring becomes a chiral center, as shown in Figs. 9 and 10; (b) the diamine ring is chiral by virtue of the value of the N–C–C–N torsion angle, as shown numerically below as well as in Figs. 9 and 10.

(Color online.) ZIFHEB. The Co(1) cation has no symmetry elements at all even when in solution where the en-like ring could flatten out by torsional motions because N1 and N2 differ by the presence of the phenyl ring that renders N2 chiral upon binding to the metal. The dissymmetry defined by the clockwise-anti-clockwise motion in going from N1 to N2 is Δ in this case; in the Co(2) cation, it is the opposite, as shown in Fig. 10.
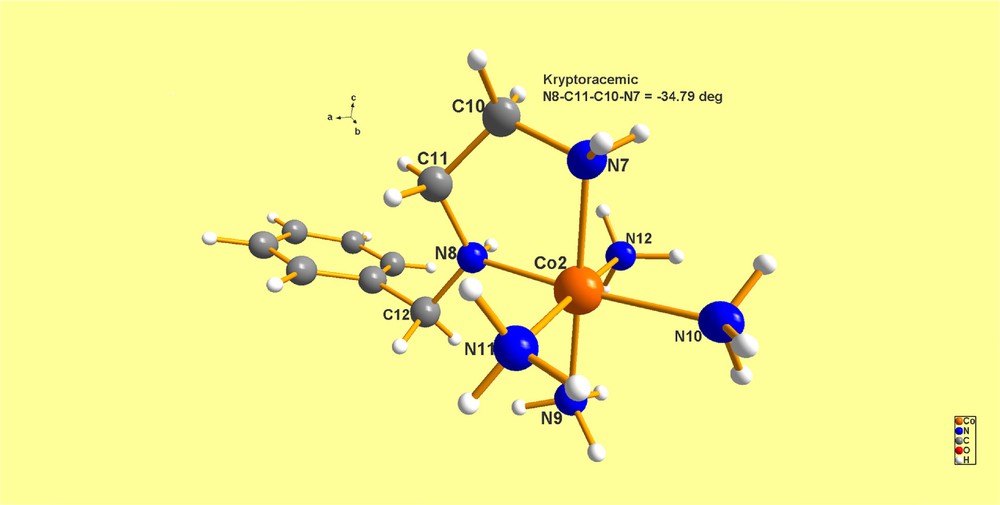
(Color online.) ZIFHEB. In this case the cation is Λ, as opposed to the Δ dissymmetry of Co(1); also, the torsion N–C–C–N angle is opposite in sign, thus constituting a kryptoracemic pair.
Centroid of Co cations = 0.75625, 0.24999, 0.24854, which is very close to ¾, ½, ½.
Atomic chirality: Co(1) has N(2) = (S) and Co(2) has N(8) = (R):
Torsion angles: Co(1) = N1–C1–C2–N2 = 50.49°.
Co(2) = N7–C10–C11–N8 = 34.79°.
As a result, the two independent Co(III) cations have chiral centers (N) and a diastereoisomeric fragment; namely, the four-membered di-amine ring. But, the crystals are exact enantiomers if one dismisses the numerical value of the torsion angles and only concentrates on the sign. This is a valid caveat inasmuch as it is expected that relatively low-energy-barrier-to-distortion fragments will undergo angular changes when they occupy different lattice environments, given that the packing forces are anisotropic.
3 Conclusions
Though apparently quite rare, the fact of the matter is that a very reliable structural study (TIQHOR) is an example of a substance that is a conglomerate, a kryptoracemate, and an unbalanced crystal, at least under the conditions of the crystallization procedure used by the authors of that study [12]. Equally novel are the examples of the last two compounds described above in that they too exhibit two different stereochemical characteristics in a Sohncke space group; i.e., they are conglomerates as well as kryptoracemates. Finally, it is almost certain there are additional examples of the above modes of crystallization already in the literature; the problem, however, is to identify them.
The facts described above suggest that the definitions of conglomerate and kryptoracemic crystallizations, enunciated many years ago, when examples such as given herein were unknown, are in need of reviewing by a qualified, international body. Such a body should:
- • retain the older definitions, in which case substances such the ones above retain multiple labels for their mode(s) of crystallization;
- • modify the definitions in order to take into account modern realities, such as the fact that the definition of conglomerate crystallization, dating back to the 19th century required that “one large single crystal be selected, dissolved, and shown to rotate the plane of polarized light”. In those days, there were no femtosecond lasers; therefore, great stereochemical rigidity was implied inasmuch as the preparation of a solution, followed by a test with a polarimeter, required rigidity lasting minutes, at least. Now, with a femtosecond pulsed laser, the time scale has been seriously changed. Do we take that into account, or ignore current facts? It is time to decide!
1 CSD: Cambridge Crystallographic Structural Database CCSD, Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK, release 1.15. They can be contacted at http://www.ccdc.cam.ac.uk.


