1 Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) belong to the group of drugs with anti-inflammatory, analgesic and antipyretic activity, which are important in the treatment of many diseases [1,2]. Paracetamol is one of the most frequently used drugs belonging to this group. It contains an active substance, N-acetyl-p-aminophenol (PAR) [3,4], presented in Fig. 1. In contradiction to other non-steroidal anti-inflammatory drugs, PAR shows very small anti-inflammatory activity and does not provoke blood clotting [5]. It is a component of various medicaments used in the treatment of cold, flue, and pain [6]. The average content of PAR in a one-component pill or capsule is 500 mg, while in a complex formulation it is most often 325 mg [5]. PAR applied in therapeutic doses is believed to be safe. Thus, it can be used by pregnant and breastfeeding women. PAR does not cause a risk of spontaneous abortion. Moreover, PAR is discharged with mother's milk at concentrations similar to its concentration in serum. This drug plays a unique role in the treatment of neonates and infants because it does not influence platelet aggregation or the rate of ductus arteriosus closing and gastric mucosa [7]. PAR is also recommended for patients with ulcer diseases, aspirin-induced asthma [8–11] and coagulability disorders [8]. However, it should be mentioned that PAR overdose could result in the accumulation of toxic metabolites, which can have serious adverse effects, and sometimes can lead to hepatic necrosis [12–16] and nephrotoxicity [17].
Chemical structure of N-acetyl-p-aminophenol.
Due to the common application of PAR in various drugs, its determination and the knowledge of its electrochemical behaviour in aqueous solutions as well as in body fluids are very important. PAR determination has been carried out through spectrophotometry [18,19], high-performance liquid chromatography [20–23], titration [24], capillary electrophoresis [25], and chemiluminescence [26]. In the past years, electroanalytical methods have attracted much attention especially in drug analysis [27–30]. They have several advantages over other analytical methods, including, among others [31–39]:
- • no need for excessive sample preparation;
- • instrumentation simplicity and portability (facile miniaturization);
- • high accuracy, sensitivity, selectivity and reproducibility;
- • fast response;
- • cost effectiveness (low power requirements);
- • possibility of detection of multiple analytes without separation steps;
- • possibility of biological matrices analysis (sweat, urine, serum, cell culture media, etc.).
These advantages are especially important in pharmaceutical analysis. Low detection and quantification limits make it possible to determine not only pharmaceuticals, but also their metabolites at nano- and picograms [38]. Moreover, drugs usually contain not only active substances (e.g., PAR), but also auxiliary components typical of monocomponent pharmaceuticals. Electroanalytical methods allow the simultaneous determination of several analytes in one sample. Among electroanalytical methods, voltammetry seems to be particularly suitable for the determination of various drugs in body fluids and the characterisation of their electrochemical behaviour [39]. Voltammetric methods allow the determination of parameters such as anodic and cathodic peak currents, total charge below peaks, oxidation and reduction potentials (half-wave potential), etc. These parameters can be used not only in the qualitative analysis of drugs and their metabolites, but also for the assessment of their antioxidant and redox properties. Additionally, voltammetric methods are important in the determination of electrode reaction mechanism for PAR and their metabolic products [40–43]. Redox properties of active substances can be useful in the evaluation and prediction of their metabolism [38,44].
For the electrochemical determination and characterisation of various compounds, especially organics, the selection of a working electrode material is critical to experimental results and success. During the selection, several factors, such as [45] redox behaviour with the analyte, reproducible electron-transfer without electrode fouling, wide potential window over which an electrode performs in a given electrolyte solution, easy surface renewal after the measurement and material toxicity and cost, should be taken into consideration. Various electrode materials, i.e. mercury, solids, and modified electrodes are applied for electroanalytical measurements. However, platinum seems to be one of the most suitable electrode materials due to its good electrochemical inertness and its ease of fabrication in many forms. In the case of drugs, glassy carbon, C60-modified glassy carbon and fullerene-C60-modified glassy carbon are often suggested for electrochemical analysis [39,40,43,46].
The aim of this work was to determine the electrochemical behaviour of PAR at a platinum electrode. The application of a Pt electrode is the initial stage in our investigations in comparison with other electrode materials that will be used in PAR electro-oxidation. The effect of scan rate, substrate concentration and pH on PAR electrode reactions was investigated. Cyclic and differential pulse voltammetry techniques were applied in our investigations.
2 Materials and methods
2.1 Chemicals and solutions
N-Acetyl-p-aminophenol (PAR) was purchased from Sigma–Aldrich (Germany). Its aqueous solutions were prepared by dissolving the substrate in the supporting electrolyte (0.1 mol/L NaClO4, Fluka, France). The concentration of PAR solutions varied in the range from 0.2 × 10−3 to 5 ×10−3 mol/L. The solutions used for the determination of the pH effect on PAR electro-oxidation were prepared by dissolving the substrate in phosphate buffer solutions (0.1 mol/L) of different pH values, prepared from stock solutions of H3PO4, NaH2PO4, Na2HPO4, and NaOH (POCH Gliwice, Poland). Doubly distilled water was used throughout the experiments. All the reagents used were of analytical grade.
2.2 Measurement methods
Electroanalytical measurements were carried out using an Autolab PGSTA30 Electrochemical Analyzer (EcoChemie, The Netherlands). A three-electrode electrochemical cell employed in measurements consisted of a reference electrode, an auxiliary electrode (platinum wire) and a platinum working electrode with a geometric surface area of 0.5 cm2. The potential of the working electrode was measured vs. the saturated calomel electrode (SCE). Before measurements in PAR solution, the Pt electrode was rinsed thoroughly in deionised water. Next, the electrode was electrochemically cycled in H2SO4 (0.5 mol/L) ten times between −0.25 V and 1.0 V (vs. SCE) with a scan rate of 100 mV/s. If unchanged CV characteristics of the Pt electrode were observed, then the electrode was removed, rinsed with deionised water and placed in the PAR solution. The determination of PAR and kinetic parameters of its electrode reactions was performed using cyclic (CV) and differential pulse (DPV) voltammetry. Cyclic voltammograms were recorded from 0 to 1.0 or 1.4 V with various scan rates (0.01 to 0.5 V/s). Differential pulse voltammograms were recorded in the same potential range with a modulation amplitude of 25 mV, a pulse width of 50 ms (scan rate: 0.01 V/s).
Before the measurements, the solutions were purged with argon in order to remove dissolved oxygen. During the measurements, an argon blanket was kept over the solutions. All experiments were carried out at room temperature.
The pH of the buffer solutions was measured using a digital pH meter (Model CPC-401, Elmetron, Poland).
3 Results and discussion
3.1 Electrochemical behaviour of PAR at Pt electrode
The electrochemical oxidation of PAR was investigated at the platinum electrode using CV and DPV methods. Examples of voltammograms are presented in Fig. 2. The electro-oxidation of PAR was investigated in the potential range from 0 to 1.0 V, in which a supporting electrolyte (0.1 mol/L NaClO4) showed no peaks (Fig. 2, curve 3). The voltammograms presented in Fig. 2 (curves 1 and 2) show that PAR is oxidized probably irreversibly in at least one electrode step at potentials lower than the potential at which oxygen evolution starts. In order to check if the substrate is adsorbed at the electrode, differential pulse voltammograms (DPV) were recorded. In this technique, the current is sampled before pulse application and at the end of the pulse. This allows diminishing surface effects like adsorption on the electrode [40]. The value of Ep determined from DPV·should corresponds to E1/2. Moreover, DPV is characterized by higher resolution than CV. One peak appearing on the DP voltammogram (Fig. 2, curve 2) proves the lack of adsorption and the diffusive character of PAR electro-oxidation.
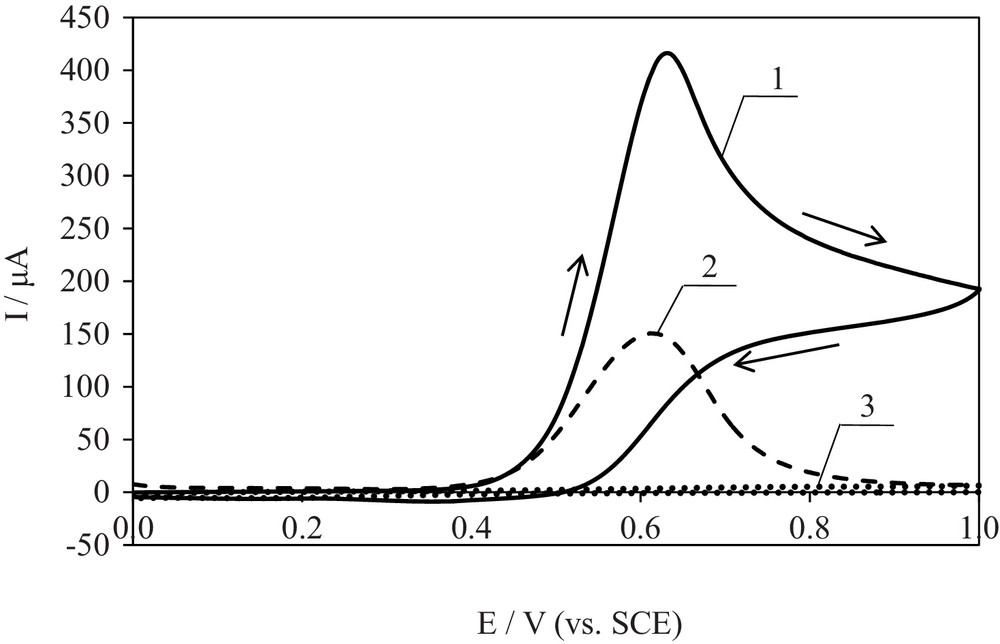
Voltammograms of PAR electro-oxidation at a Pt electrode: curve 1, CV; 2, DPV; 3, CV recorded in the supporting electrolyte (c = 5 × 10−3 mol/L in 0.1 mol/L NaClO4, v = 0.01 V/s).
The half-wave potential (E1/2) of PAR electro-oxidation determined from the cyclic voltammogram (CV) totals 0.59 V and corresponds to the peak potential (Epa) determined from the differential pulse voltammogram (DPV). The value of E1/2 was shifted towards more positive values with increasing the scan rate, showing the increasing irreversibility of the process.
3.2 An effect of scan rate
PAR as well as acetylsalicylic (ASA) and salicylic (SA) acids is an active component of NSAIDs. In our previous studies [30], we proved the effect of the scan rate on ASA and SA oxidation. Thus, further investigations of PAR electro-oxidation included the determination of the scan rate effect. The scan rate effect was investigated in the range from 0.01 to 0.5 V/s. Examples of cyclic voltammograms recorded at different scan rates are presented in Fig. 3. An increase in the scan rate results in an increase in the peak current determined for PAR electro-oxidation. In the reverse scan, a cathodic peak appears at higher scan rates. Its current also increases with increasing the scan rate. This proves the reduction of products formed during PAR electro-oxidation. Potentials and currents were determined for anodic (Epa, ipa) and cathodic (Epc, ipc) peaks from cyclic voltammograms.
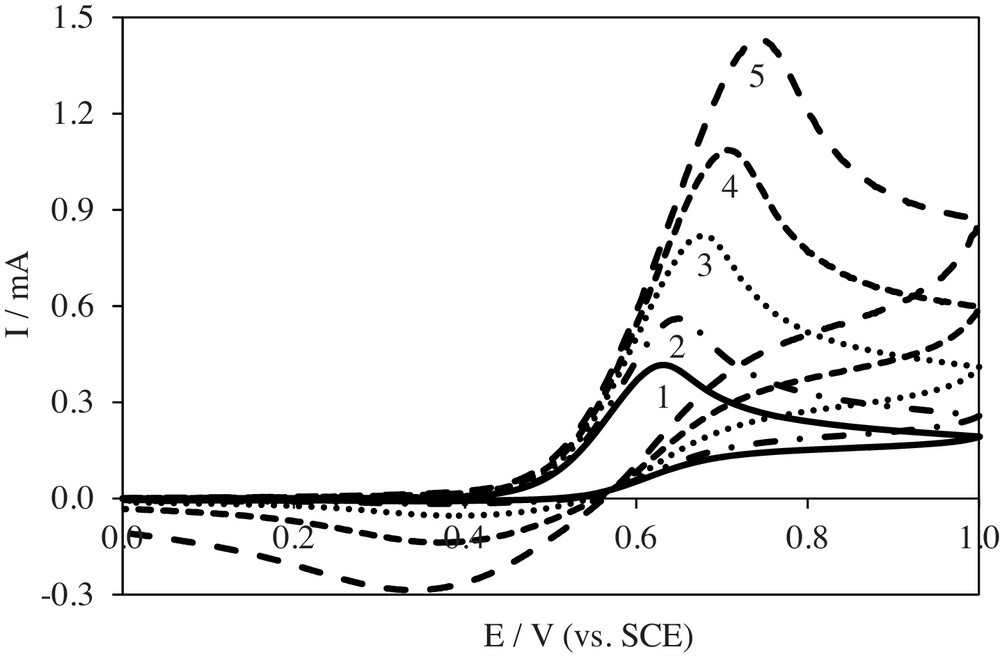
Cyclic voltammograms of PAR electro-oxidation recorded at Pt electrode at different scan rates (c = 5.0 × 10−3 mol/L in 0.1 mol/L NaClO4): curve 1, 0.01 V/s; 2, 0.02 V/s; 3, 0.05 V/s; 4, 0.1 V/s; 5, 0.2 V/s.
In order to characterize the electrode reaction of PAR electro-oxidation, two relationships were determined: ip vs. the square root of the scan rate (v1/2) and logarithm of ip vs. the logarithm of the scan rate (ln v). These relationships make it possible to state whether the electrode reaction is controlled by adsorption or diffusion of the substrate to the electrode surface. Fig. 4 shows such dependences determined for the first step of PAR electro-oxidation in NaClO4. If the electrode reaction is controlled by diffusion, the dependence of ip vs. v1/2 is linear and intercepts the origin. If this dependence does not cross the origin, the electrode reaction can be controlled by adsorption [47–53]. In the scan rate range from 0.01 to 0.5 V/s, the anodic peak currents of PAR electro-oxidation and cathodic peak currents recorded for the products formed during the electro-oxidation process depend linearly on the square root of the scan rate (Fig. 4A) and are described by the following equations:
| ipa (mA) = {2.377[v(V·s−1)]1/2} + 0.203, R2 = 0.993 | (1) |
| ipc (mA) = {–0.727[v(V·s−1)]1/2} + 0.098, R2 = 0.982 | (2) |

A. Dependence of anodic (ipa) and cathodic (ipc) peak currents on the square root of the voltage scan rate (v). B. Dependence of ipa and ipc on v in double logarithm coordinates for PAR oxidation. C. Variation of the scan rate-normalized current (ipa/v1/2) with the scan rate; 5.0 × 10−3 mol/L in 0.1 mol/L NaClO4 at a Pt electrode.
The dependence described by equation (1) does not intercept the origin, which could indicate that the electrode reaction is adsorption controlled. However, a linear dependence of ln ip vs. ln v, which is presented in Fig. 4B and is described by equation (3):
| ln ipa (mA) = {0.396 ln v(V·s−1)} + 0.900, R2 = 0.999, | (3) |
does not confirm such a conclusion. The slope of this linear dependence equals 0.396 and indicates that the reaction of PAR electro-oxidation is diffusion controlled. If the slope is equal or close to 0.5, this means a diffusion control of the electrode process. Otherwise, if the slope is equal or close to 1.0, then the process is controlled by adsorption [51,52].
The slope of the ln ip vs. ln v dependence curve (eq. (4)) describing the reduction of PAR electro-oxidation products (the reverse scan) is higher (1.198) and indicates an adsorption controlled process.
| ln |ipc| (mA) = {1.198 ln v(V·s−1)} + 0.272, R2 = 0.985 | (4) |
Further analysis of the PAR electro-oxidation (the anodic peak) included the determination of ipa/v1/2 vs. v dependence for PAR electro-oxidation. This dependence is presented in Fig. 4C. It is clear that the scan rate-normalized current (ip/v1/2) depends on v. If the process is reversible or irreversible without preceding or following the chemical reaction, the dependence on v would not be observed [42]. The shape of this dependence (Fig. 4C) for the anodic peak of the PAR electro-oxidation is typical for an EC mechanism [51–54].
An effect of the scan rate on the potential of PAR electro-oxidation was also investigated. Fig. 5A presents dependences of the peak potential (Ep) vs. the scan rate (v) for electro-oxidation of PAR and electro-reduction of PAR electro-oxidation products. These dependences show that the anodic and cathodic peak potentials shift towards more positive and more negative potentials, respectively, if the scan rate increases. This proves the irreversible character of both processes. Moreover, the value of the electron-transfer coefficient for the reaction of PAR electro-oxidation was calculated according to the following equation [53,55–56]:
| (5) |

A. Dependence of anodic (Epa) and cathodic (Epc) peak potential on the scan rate (v) for the oxidation of PAR in 0.1 mol/L NaClO4 at the Pt electrode. B. Dependence of Epa and Epc on ln v; c = 5.0 × 10−3 mol/L.
If the process is irreversible, as in the case of PAR oxidation, then ip can be described by the following equation [53]:
| (6) |
| (7) |
The dependence of ln ip vs.
| ln ip (mA) = {9.26 [(Ep – Eo)]} – 0.896, R2 = 0.987 | (8) |
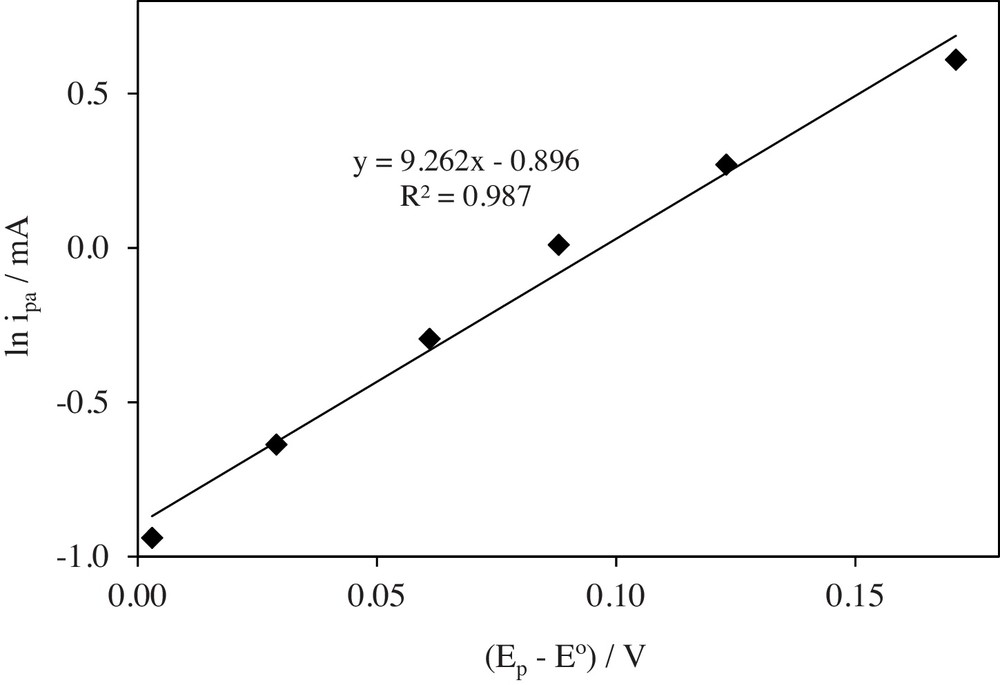
Variation of ln ipa vs. (Ep – E°) for the oxidation of PAR at a Pt electrode in 0.1 mol/L NaClO4.
Its slope equals 9.26. The slope was used in calculation of anodic transfer coefficient, which is equal to 0.28. The diffusion coefficient of PAR was calculated using the following equation, which is appropriate for irreversible reactions [53,57–59]:
| (9) |
| (10) |
3.3 The effect of PAR concentration and pH on the substrate electro-oxidation
The effect of PAR concentration in 0.1 mol/L NaClO4 on its electro-oxidation was investigated in the range from 0.2 × 10−3 to 5.0 × 10−3 mol/L. CV curves were recorded using a scan rate of 0.01 V/s. Changes in anodic peak currents observed in PAR electro-oxidation were linear in the investigated concentration range. Examples of voltammograms recorded for different substrate concentrations are presented in Fig. 7.

Cyclic voltammograms of PAR electro-oxidation recorded at a Pt electrode at various substrate concentrations: curve 1, 0.5 mmol/L; 2, 1 mmol/L; 3, 2 mmol/L; 4, 3 mmol/L; 5, 4 mmol/L. The inset shows the dependence of the anodic current on the PAR concentration; v = 0.01 V/s.
The dependence of ipa on the PAR concentration is described by the following equation:
| ipa (μA) = {79.60 [c (mmol/L)]} – 2.170, R2 = 0.999 | (11) |
Due to the linearity of ip vs. the PAR concentration, the cyclic voltammetry method can be successfully applied to the determination of PAR concentration [61,62]. Cyclic voltammograms recorded for different substrate concentrations were used for the determination of the peak potential (Epa), the half-peak potential (Epa/2) and the half-wave potential (E1/2). On the basis of the determined values, the anodic transfer coefficient (βnβ) and heterogeneous rate constant (kbh) were calculated according to the equations presented in papers [50] and [63]. The anodic transfer coefficient for PAR electro-oxidation is equal to 0.31 ± 0.05. The heterogeneous rate constant determined at the half-wave potential is equal to (2.01 ± 0.05) × 10−4 cm/s.
The effect of pH on PAR electro-oxidation was investigated in the pH range from 2 to 13. The dependences of Epa and E1/2 on pH are presented in Fig. 8.

Effect of pH on Epa (curve 1) and E1/2 (curve 2) for PAR electro-oxidation.
Both of them are linear and they are described by the following equations:
| Epa (V) = 0.781 – 0.033 pH, (R2 = 0.996) | (12) |
| E1/2 (V) = 0.744 – 0.036 pH, (R2 = 0.998) | (13) |
Taking into consideration the equation [42,64]:
| (14) |
Taking into consideration the results of the investigations as well as the mechanism of PAR electro-oxidation (Scheme 1) at other electrodes suggested in papers [39,41,65–68], it can be concluded that the first step of the process includes the exchange of two electrons and one proton resulting in the formation of N-acetyl-p-quinoneimine. This step is followed by the chemical reaction resulting in the formation of p-quinoneimine. However, this conclusion should be confirmed by further investigations leading to the determination of intermediate products of PAR electro-oxidation with the application of spectroscopic and chromatographic methods. The results will be described in the next paper.
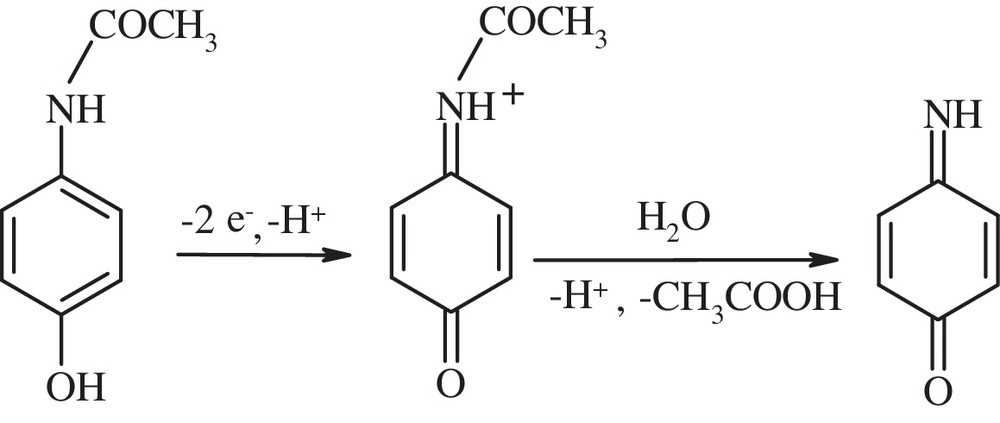
Mechanism of PAR electro-oxidation.
4 Conclusions
The electrochemical behaviour of PAR at a Pt electrode in an aqueous solution proceeds in at least one electrode step before the potential reaches the value at which oxygen evolution starts. The half-wave potential of PAR electro-oxidation was determined from cyclic voltammograms and is equal to 0.59 V. Its value corresponds to the peak potential determined from differential pulse voltammograms.
The dependence of the anodic peak current vs. PAR concentration is linear and can be applied to the determination of the substrate concentration in environmental samples and pharmaceuticals. The calculated parameters of PAR electro-oxidation are as follows: anodic charge transfer coefficient, 0.31; diffusion coefficient, (7.63 ± 0.05) × 10−6 cm2/s; heterogeneous rate constant, (2.01 ± 0.05) × 10−4 cm/s (determined at E1/2).
In the tested pH range, the dependence of Epa and E1/2 vs. pH is linear. The process of PAR electro-oxidation includes the exchange of two electrons and one proton in the first step. This step is followed by the chemical reaction. Thus, the investigated process proceeds according to the EC mechanism.



Vous devez vous connecter pour continuer.
S'authentifier